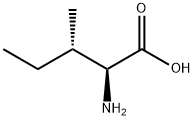
L-Isoleucine synthesis
- Product Name:L-Isoleucine
- CAS Number:73-32-5
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
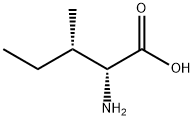
1509-35-9
310 suppliers
$8.00/250mg

73-32-5
902 suppliers
$5.00/250mg
Yield:-
Reaction Conditions:
with ammonium metavanadate;salicylaldehyde;sodium hydroxide in water; pH=11; for 6 h;Reflux;
Steps:
2.1. Epimerization of isoleucine
General procedure: A round-bottom flask was loaded with isoleucine (1.31 g, 10 mmol), NaOH (0.4 g, 10 mmol) and water (5 mL). The mixture was gently heated until dissolution. Salicylaldehyde (0.011 mL, 0.1 mmol) and NH4VO3 (0.0112 g, 0.1 mmol) were added and the yellow solution (pH = 11) was heated under reflux 6 h. After this period the heating was carefully continued without reflux to evaporate approx. 1/2 of the solvent. Then the solution was cooled to room temperature, 20 mL of EtOH and 0.7 mL of acetic acid were added. The mixture was cooled in a freezer below 0 °C, white precipitate of isoleucine was filtered off, washed with EtOH and dried in air.
References:
Krivosudsky, Luká?;Schwendt, Peter;Filo, Juraj [Catalysis Communications,2016,vol. 86,p. 96 - 99]
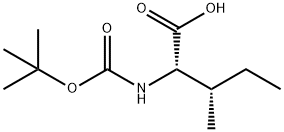
13139-16-7
322 suppliers
$11.00/5G

73-32-5
902 suppliers
$5.00/250mg