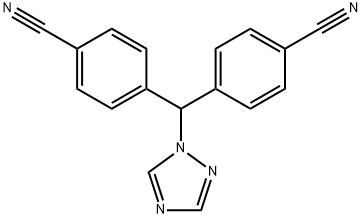
Letrozole synthesis
- Product Name:Letrozole
- CAS Number:112809-51-5
- Molecular formula:C17H11N5
- Molecular Weight:285.3

288-88-0
778 suppliers
$10.00/5g
![Benzonitrile, 4,4'-[[[(4-methylphenyl)sulfonyl]oxy]methylene]bis-](/CAS/20210305/GIF/935477-95-5.gif)
935477-95-5
1 suppliers
inquiry

112809-51-5
662 suppliers
$39.00/10mg
Yield:112809-51-5 97.42%
Reaction Conditions:
Stage #1: 1,2,4-Triazolewith tetrabutylammomium bromide;potassium carbonate in water;4-methyl-2-pentanone; for 2 h;
Stage #2: toluene-4-sulfonic acid bis-(4-cyano-phenyl)-methyl ester in water;4-methyl-2-pentanone at 20 - 30; for 48 h;
Stage #3: with hydrogenchloride in water;4-methyl-2-pentanone; for 1 h;
Steps:
4
EXAMPLE 4; PREPARATION OF LETROZOLE OF FORMULA I 700 ml of water was taken into a round bottom flask to which was then added 49.8 g of 1,2,4-triazole with stirring for about 5 minutes, and then 99.6 g of potassium carbonate was added with stirring for 10 minutes. 350 ml of methyl isobutyl ketone (MIBK) and 58.1 g of tetrabutylammonium bromide were charged and stirred for 2 hours. 70 g of toluene sulphonic acid bis-(4-cyano phenyl)methyl ester was charged to the obtained reaction mixture at 20-30° C. and stirred for 48 hours. The formed aqueous and organic layers were separated and the aqueous layer extracted with 140 ml of methyl isobutyl ketone (MIBK) followed by washing the obtained organic layer with 700 ml of water. 1050 ml of 5N hydrochloric acid was charged to the organic layer, and stirred for 60 minutes. The formed aqueous and organic layers were separated and the organic layer extracted with 140 ml of aqueous 5N hydrochloric acid followed by washing the aqueous layer with 2×40 ml of n-hexane. The aqueous layer was basified with 350 g of sodium carbonate to get a pH of about 8.5 at a temperature below 25° C. followed by extraction with 2×350 ml of dichloromethane and the organic layer was washed with 700 ml of water. The resultant organic layer was distilled completely at below 40° C. and 350 ml of methanol and 1050 ml of water were charged, and stirred for 1 hour at 10-15° C. The formed precipitate was filtered followed by washing the solid with 350 ml of water, and suction drying of the solid for 30 minutes to afford 97.42% pure title compound. Dissolved the above-obtained letrozole compound in 140 ml of dichloromethane. A silica gel bed was prepared, using 140 g of silica gel (230-400 mesh) on a fritted glass filter funnel (10 cm height and 10 cm diameter), and the silica bed was washed with 1.4 L of dichloromethane. The dichloromethane solution was passed through the silica gel bed and the bed washed with 28 L of dichloromethane. The obtained filtrate was concentrated below 40° C. to a volume of about 140 ml of dichloromethane then 500 ml of methanol was charged and stirred for 10 minutes. The obtained solution was concentrated below 40° C. to a volume of 105-120 ml and 350 ml of water was charged and stirred for 1 hour. The formed solid was filtered followed by washing the solid with 140 ml of water and drying the solid at 55-60° C. for 4 hours under reduced pressure to afford 11.4 g of title compound having purity by HPLC of 99.8% and all other impurities 0.15%. Impurities: Letrozole related compound A of Formula VII: not detected. 4,4',4-methylidenetrisbenzonitrile of Formula IX: not detected. 4,4'-dicyanobenzophenone of Formula III: not detected. 4,4'-(hydroxy methylene)bis benzonitrile of Formula IV: not detected. Methane sulfonic acid bis-(4-cyano-phenyl)-methyl ester of Formula X: not detected. Highest unidentified impurity: 0.05%
References:
US2007/100149,2007,A1 Location in patent:Page/Page column 7

288-88-0
778 suppliers
$10.00/5g

112809-57-1
38 suppliers
inquiry

112809-51-5
662 suppliers
$39.00/10mg
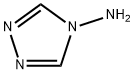
584-13-4
476 suppliers
$5.00/5g
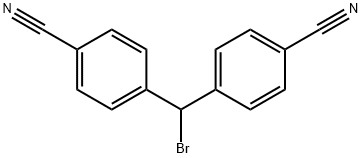
69545-39-7
40 suppliers
inquiry

112809-51-5
662 suppliers
$39.00/10mg
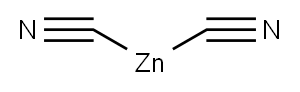
557-21-1
88 suppliers
$12.00/5g

102994-04-7
11 suppliers
inquiry

112809-51-5
662 suppliers
$39.00/10mg
290-87-9
237 suppliers
$9.00/1g
![Benzonitrile, 4,4'-[[2-(1-methylethylidene)hydrazinyl]methylene]bis-](/CAS/20210305/GIF/1280504-38-2.gif)
1280504-38-2
1 suppliers
inquiry

112809-51-5
662 suppliers
$39.00/10mg