Linezolid synthesis
- Product Name:Linezolid
- CAS Number:165800-03-3
- Molecular formula:C16H20FN3O4
- Molecular Weight:337.35
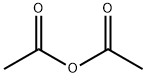
108-24-7
5 suppliers
$14.00/250ML
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
202 suppliers
$7.00/100mg

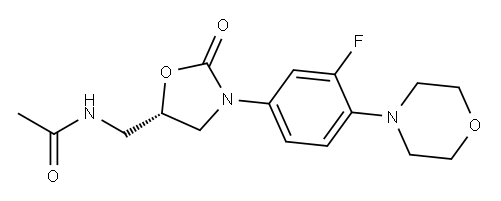
165800-03-3
734 suppliers
$5.00/100mg
Yield:165800-03-3 98%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 3 h;
Steps:
8. Synthesis of compound 1, Linezolid
Compound 9 (100 mg, 0.34 mmol) was dissolved into 5 mL of anhydrous dicholoromethane in a 25 mL round bottom flask. The mixture was treated with Ac2O (0.2 mL, 1.59 mmol) and TEA (0.28 mL, 2.0 mmol) at room temperature. After 3 hours, dilute the mixture with EtOAc (40 mL).Wash the mixture with HCl (0.1 M, 5 mL), Sat. NaHCO3 (10) and brine (10 mL).Dry the organic layer with Na2SO4. Filter the organic layer and concentrate the organic layer at reduced pressure, the desired product was purified by silica gel column. Yield, 129 mg, 98%. 1H NMR (400 MHz, DMSO-d6) δ 8.27 (s, 1H), 7.49 (d, J = 15.0 Hz, 1H), 7.18 (d, J = 8.6 Hz, 1H), 7.04 (t, J = 9.2 Hz, 1H), 4.72 (s, 1H), 4.08 (t, J = 8.8 Hz, 1H), 3.71 (m, 4H), 2.95 (m, 4H), 1.85 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 170.51, 156.26, 154.51, 153.83, 135.96 (d, J = 8.8 Hz), 133.89 (d, J = 10.6 Hz), 119.60 (d, J = 4.0 Hz), 114.42 (d, J = 2.6 Hz), 107.16, 106.90, 72.03, 66.61, 51.14, 47.73, 41.88, 22.87. 19F NMR (376 MHz, DMSO) δ -121.26.
References:
Wang, Keyin;Chen, Yinzhe;Wang, Siyuan;Zhang, Qian [Tetrahedron Letters,2022,vol. 105,art. no. 154050] Location in patent:supporting information
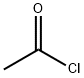
75-36-5
559 suppliers
$17.92/100G
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
202 suppliers
$7.00/100mg

165800-03-3
734 suppliers
$5.00/100mg

108-24-7
5 suppliers
$14.00/250ML
![(R)-5-(Azidomethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone](/CAS2/GIF/168828-84-0.gif)
168828-84-0
113 suppliers
inquiry

165800-03-3
734 suppliers
$5.00/100mg

168828-89-5
99 suppliers
$150.00/10mg

108-24-7
5 suppliers
$14.00/250ML

165800-03-3
734 suppliers
$5.00/100mg

1027135-00-7
3 suppliers
inquiry

183805-10-9
28 suppliers
inquiry

165800-03-3
734 suppliers
$5.00/100mg