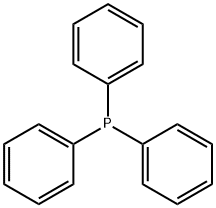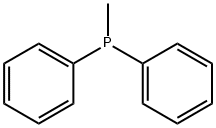
METHYLDIPHENYLPHOSPHINE synthesis
- Product Name:METHYLDIPHENYLPHOSPHINE
- CAS Number:1486-28-8
- Molecular formula:C13H13P
- Molecular Weight:200.22

917-64-6
146 suppliers
$45.00/10ml
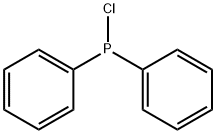
1079-66-9
425 suppliers
$5.00/5g

1486-28-8
157 suppliers
$9.00/1g
Yield:1486-28-8 74%
Reaction Conditions:
in diethyl ether at -35;Inert atmosphere;
Steps:
A solution of CH3MgIin Et2O(20 mL) was added dropwiseat - 35 °C to a solution of chlorodipenylphosphane (29.8 g,135.06 mmol) in Et2O(100 mL) at - 35 °C under N2andthe mixture was stirred for overnight. After filtration, theorganic phase was washed with distilled water (3 × 10 mL),separated and dried with anhydrous Na2SO4.Methyldiphenylphosphine(20 g, 99.90 mmol) was obtained as a colorlessliquid by reduced pressure distillation in 74% yield. 1H NMR(400 MHz, CDCl3,298 K): δ 1.6 (d, 3H, CH3),7.3-7.7 (m,10H, Ph). 31P NMR (162 MHz, CDCl3,298 K): δ - 26.8 (s).A solution of n-BuLi (21.5 mL, 2.4 mol/L in n-hexane,51.6 mmol) in n-hexane (30 mL) was added dropwise to asolution of methyldiphenylphosphine (10 g, 49.95 mmol)and TMEDA (6.1 g, 25 mL) in n-hexane (15 mL) at below0 °C. The reaction mixture was then stirred for 60 h underN2at room temperature. The yellow precipitate was filteredoff and washed with n-hexane (3 × 20 mL) to give (diphenylphosphino)methyllithium-TMEDA (16 g, 49.6 mmol,99% yield).
References:
Wei, Wei;Yu, Buwei;Alam, Fakhre;Huang, Yongwang;Cheng, Shaoling;Jiang, Tao [Transition Metal Chemistry,2019,vol. 44,# 2,p. 125 - 133]

13639-74-2
0 suppliers
inquiry

1486-28-8
157 suppliers
$9.00/1g

2129-89-7
116 suppliers
$11.00/5g

1486-28-8
157 suppliers
$9.00/1g