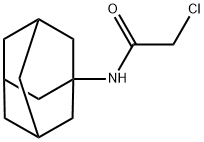
N-ADAMANTAN-1-YL-2-CHLORO-ACETAMIDE synthesis
- Product Name:N-ADAMANTAN-1-YL-2-CHLORO-ACETAMIDE
- CAS Number:5689-59-8
- Molecular formula:C12H18ClNO
- Molecular Weight:227.73

768-95-6
412 suppliers
$10.00/5g

107-14-2
273 suppliers
$15.00/25g

5689-59-8
50 suppliers
$60.00/250mg
Yield:5689-59-8 99.6%
Reaction Conditions:
Stage #1: 1-adamanthanol;chloroacetonitrilewith sulfuric acid in acetic acid;N,N-dimethyl-formamide at 20 - 80;
Stage #2: with water in acetic acid;N,N-dimethyl-formamide at 70;
Steps:
12 Example 12
A 0.50 l round-bottom three-necked flask, equipped with reflux condenser, oil ventile, mechanical stirrer, thermometer, and dropping funnel is charged with 1-hydroxyadamantane (10.00 g, 65.69 mmol), chloroacetonitrile (9.92 g, 131.38 mmol), dimethylformamide (11 ml) and acetic acid (21 ml, 363.96 mmol). The resulting suspension is slowly treated with sulphuric acid (96 %, 21 ml) at room temperature with vigorous stirring upon which the reaction mixture reaches approximately 80 °C. After completion of addition the clear reaction mixture is slowly treated with water (15 °C, 118 ml) at 70 °C. Upon ceasing of the exothermic reaction the product crystallizes. The resulting suspension is cooled to 0 - 5 °C with stirring. After filtration, the colourless crystalline solid is washed in a stream of water and dried under reduced pressure (40 °C, 19 h, 25 mbar) to yield 14.90 g (65.43 mmol, 99.60 %) of N-Adamantan-1-yl-2-chloro-acetamide. 1H NMR (300 MHz, CDCl3, ppm): 6.24 (b,1H, NH), 3.94 (s, 2H, RCH2Cl), 2.10 (m, 3H, R3CH), 2.03 (m, 6H, RCH2R), 1.70 (m, 6H, RCH2R). 13C NMR (75 MHz, DMSO-d6, ppm): δ 164.61 (CO), 52.39, 42.89, 41.37, 36.22, 29.38. MS (EI, m/z): 227 [M]+. IR (KBr, cm-1): 1662 s (νRCONHR, Amide I), 1569 s (Amide II). mp.: 123.4 °C.
References:
EP1820792,2007,A1 Location in patent:Page/Page column 7
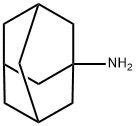
768-94-5
393 suppliers
$41.00/25g

79-04-9
350 suppliers
$12.00/5g

5689-59-8
50 suppliers
$60.00/250mg

593-71-5
297 suppliers
$6.00/5g

4411-25-0
80 suppliers
$14.14/250mgs:

5689-59-8
50 suppliers
$60.00/250mg
665-66-7
568 suppliers
$16.00/5g

79-04-9
350 suppliers
$12.00/5g

5689-59-8
50 suppliers
$60.00/250mg

768-95-6
412 suppliers
$10.00/5g
79-07-2
386 suppliers
$5.00/25g

5689-59-8
50 suppliers
$60.00/250mg