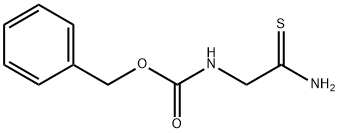
N-BENZYLOXYCARBONYLGLYCINE THIOAMIDE synthesis
- Product Name:N-BENZYLOXYCARBONYLGLYCINE THIOAMIDE
- CAS Number:49548-40-5
- Molecular formula:C10H12N2O2S
- Molecular Weight:224.28
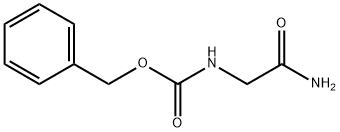
949-90-6
114 suppliers
$9.00/5g

49548-40-5
64 suppliers
$50.00/50mg
Yield:49548-40-5 79%
Reaction Conditions:
with Lawessons reagent in 1,4-dioxane at 20 - 60; for 4.5 - 4.75 h;
Steps:
A-1
Synthesis of Compound A.2. To a solution of compound A.1 (0.5 g, 0.0024 mol) in dioxane (7 mL) was added Lawesson's reagent (0.5 g, 0.0013 mol). The reaction was heated at 60° C. for 30-45 min. The reaction was brought to RT and stirred for an additional 4 hr. Dioxane was removed under reduced pressure. The reaction mixture was diluted with EtOAc (3 mL) and the organic layer was washed with sat. NaHCO3 (2 mL). The aqueous layer was again extracted with EtOAc (2×5 mL). The combined organic extracts were again washed with sat. NaHCO3 (3×5 mL), dried (Na2SO4) and concentrated under reduced pressure to furnish compound A.2 as a light yellow solid (0.42 g, 79%). 1H NMR: (CDCl3-DMSO-d6, 200 MHz) δ: 7.4 (s, 5H), 6.4 (1H, D2O exchangeable), 5.2 (s, 2H), 4.2 (d, 2H, J=5 Hz); MS: m/z 224.9 [M+1]+.
References:
Sunesis Pharmaceuticals, Inc US2009/5359, 2009, A1 Location in patent:Page/Page column 32

3589-41-1
94 suppliers
$20.00/1g

49548-40-5
64 suppliers
$50.00/50mg
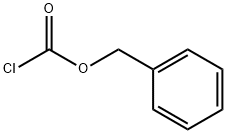
501-53-1
393 suppliers
$10.00/1g

49548-40-5
64 suppliers
$50.00/50mg
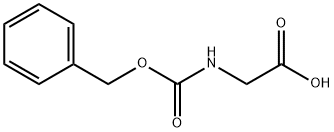
1138-80-3
442 suppliers
$6.00/10g

49548-40-5
64 suppliers
$50.00/50mg