
tert-Butyl 2-cyanopiperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 2-cyanopiperidine-1-carboxylate
- CAS Number:153749-89-4
- Molecular formula:C11H18N2O2
- Molecular Weight:210.27

388077-74-5
128 suppliers
$35.93/5g

153749-89-4
153 suppliers
$7.00/250mg
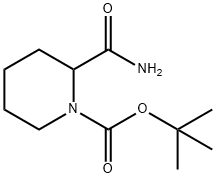
Yield:153749-89-4 97%
Reaction Conditions:
with pyridine;oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane;acetonitrile at -5 - 20;
Steps:
14.a
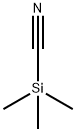
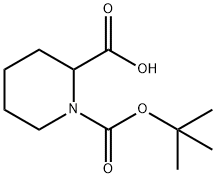
Acetonitrile (220 mL) and DMF (3.82 mL, 49.4 mmol) were added to a 500 mL round bottom flask equipped with stir bar. Cooled the mixture down to-5°C and to it added oxalyl chloride (24.7 mL, 49.4 mmol, 2 M dichloromethane). The resulting mixture was stirred for 15 min. This was followed by addition of solution of 2-carbamoyl-piperidine-1- carboxylic acid tert-butyl ester (9.4 g, 41.2 mmol) in acetonitrile. (50 mL) and pyridine (8.3 mL, 103 mmol). Reaction mixture was left stirring at room temperature overnight. The reaction mixture was concentrated in vacuo and the residue was dissolved in ethyl acetate (300 mL). The organic phase was successively washed with water (300 mL) and brine (200 mL), dried (sodium sulfate), filtered and concentrated in vacuo to isolate the title compound (8.44 g, 97%) as a yellow solid. 1H NMR (CDCIs), 8 (ppm): 5.23 (bs, 1H), 4.03 (bs, 1H), 2.93 (t, 1H), 1.75 (m, 5H), 1.46 (m, 10H).
References:
WO2005/80386,2005,A1 Location in patent:Page/Page column 42-43
7677-24-9
367 suppliers
$19.00/5g

75844-69-8
161 suppliers
$14.00/1g

153749-89-4
153 suppliers
$7.00/250mg

22821-76-7
114 suppliers
$8.00/1g
98303-20-9
242 suppliers
$11.19/5G

153749-89-4
153 suppliers
$7.00/250mg

7677-24-9
367 suppliers
$19.00/5g

1078727-67-9
0 suppliers
inquiry

153749-89-4
153 suppliers
$7.00/250mg
19158-51-1
169 suppliers
$52.00/1g

75844-69-8
161 suppliers
$14.00/1g

153749-89-4
153 suppliers
$7.00/250mg