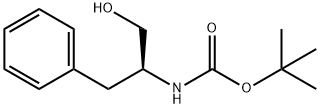
N-Boc-L-Phenylalaninol synthesis
- Product Name:N-Boc-L-Phenylalaninol
- CAS Number:66605-57-0
- Molecular formula:C14H21NO3
- Molecular Weight:251.32

51987-73-6
211 suppliers
$9.00/5g

66605-57-0
317 suppliers
$6.00/1g
Yield:66605-57-0 94%
Reaction Conditions:
with methanol;sodium tetrahydroborate;lithium chloride at 5 - 20;Inert atmosphere;
Steps:
4 Synthesis of compound 7b
Compound 8b (27.9 g, 0. eq.) and lithium chloride (8.5 g, 2.0 eq.) were added to methanol (500 ml) and dissolved with stirring under nitrogen. The mixture was cooled to below 5 ° C in an ice-water bath, sodium borohydride (7.5g, 2. Oeq.) was added in portions to the reaction solution, and the reaction mixture was incubated at room temperature. The reaction was monitored by TLC until the starting material disappeared. The reaction was placed in ice water The bath was cooled to below 5 ° C, 15 ml of water was added dropwise to the mixture, and 2N hydrochloric acid was added dropwise to a pH of 2-3. The majority of the solvent was evaporated off and the residue was adjusted to pH 9 with 2 mol / L NaOH solution. The aqueous layer was extracted with dichloromethane (30 ml x 3) and the combined organic layers were washed with water (30 ml) and saturated brine (30 ml) Sodium drying. Filtration and concentration of the filtrate under reduced pressure gave a crude yellow oil. The crude product was recrystallized from ethyl acetate n-heptane (2: 1 mixed solvent) to give compound 7b (23.6 g, yield 94%).
References:
Zhejiang Yongning Pharmaceutical Co., Ltd.;Ye Tianjian;Lu Xiuwei;Liu Yongjiang;Wang Tong;Shu Zhan CN105198829, 2017, B Location in patent:Paragraph 0073-0075
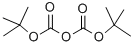
24424-99-5
819 suppliers
$13.50/25G
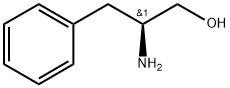
3182-95-4
452 suppliers
$5.00/5g

66605-57-0
317 suppliers
$6.00/1g
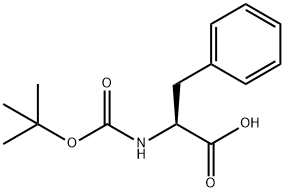
13734-34-4
531 suppliers
$5.50/5g

66605-57-0
317 suppliers
$6.00/1g
![L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-, 4,6-dimethoxy-1,3,5-triazin-2-yl ester](/CAS/20210111/GIF/1025839-59-1.gif)
1025839-59-1
0 suppliers
inquiry

66605-57-0
317 suppliers
$6.00/1g
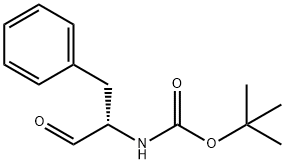
72155-45-4
163 suppliers
$22.00/250mg

66605-57-0
317 suppliers
$6.00/1g