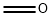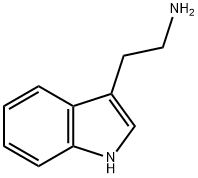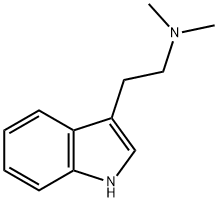
N,N-Dimethyltryptamine synthesis
- Product Name:N,N-Dimethyltryptamine
- CAS Number:61-50-7
- Molecular formula:C12H16N2
- Molecular Weight:188.27

3389-21-7
116 suppliers
$19.00/250mg
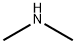
124-40-3
500 suppliers
$18.00/100ml

61-50-7
2 suppliers
$25.00/5mg
Yield:61-50-7 84%
Reaction Conditions:
in methanol at 20;
Steps:
5
To 250 mg of 3 (2- bromoethyl)indole in a round-bottomed flask, a 10 M excess of dimethyiamine (6 mL of 2M in MeOH) was added and the solution was stirred at r.t. overnight. After adding 5 mL sodium bicarbonate (2%), the reaction mixture was extracted with chloroform (3x5 ml) and back extracted with H2O (lx5mL). The combined extracts were dried over MgSO4 and evaporation of the solvent gave the product in crystalline form. Yield: 0.21 g, 84%, yellowish crystals, m.p.: 45-47 0C; TLC (EtAc:MeOH: acetic acid, 8:2:0.5 v/v/v): RF = 0.33. H NMR, δ: 10.43 (s, 1 H), 7.42-7.06 (m, 5 H), 2.68 (m, 4H), 2.26 (S, 6 H). 13C NMR (CDCl3): δ 136.5, 124.3, 123.2, 121.2, 119.7, 117.6, 113.4, 111.1, 61.7, 41.2, 22.4. Anal, calcd for C12H16N2: C, 76.55; H, 8.57; N, 14.88%. Found; C, 76.60; H, 8.70; N, 14.70%.
References:
Wisconsin Alumni Research Foundation;RUOHO, Arnold E.;HAJIPOUR, Abdol R.;CHU, Uyen B.;FONTANILLA, Dominique A. WO2010/59711, 2010, A1 Location in patent:Page/Page column 33

91566-04-0
19 suppliers
$190.00/100 mg

61-50-7
2 suppliers
$25.00/5mg

2155-94-4
174 suppliers
$14.00/25g
201230-82-2
1 suppliers
inquiry
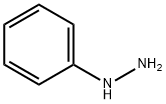
100-63-0
358 suppliers
$10.00/1g

61-50-7
2 suppliers
$25.00/5mg