
Naloxone synthesis
- Product Name:Naloxone
- CAS Number:465-65-6
- Molecular formula:C19H21NO4
- Molecular Weight:327.37
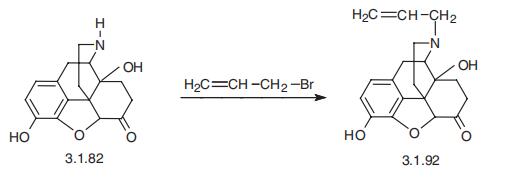
33522-95-1
82 suppliers
$60.60/013-1ml

106-95-6
416 suppliers
$10.00/5g

465-65-6
62 suppliers
$27.89/004-1ml
Yield:465-65-6 84%
Reaction Conditions:
with triethylamine in 1-methyl-pyrrolidin-2-one;water at 70; for 9.5 h;Inert atmosphere;
Steps:
7
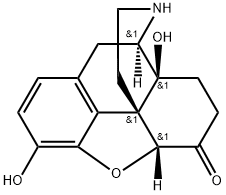
Example 7; Naloxone Allyl bromide (56 mg; 0.463 mmol) and Et3N (45 μl; 0.327 mmol) were added to a suspension of noroxymorphone (Example 5, 100 mg; 0.348 mmol) in a mixture of NMP/H2O (10:1; 0.35 mL). The reaction vessel was purged with argon and the mixture was stirred at 70° C. for 2 h. At that time, additional Et3N (45 μl; 0.327 mmol) was added and the mixture was stirred for an additional 7.5 h at 70° C. The reaction mixture was then cooled to room temperature, diluted with dichloromethane (15 mL), and washed with saturated NaHCO3 (3×3 mL). The aqueous layer was re-extracted with dichloromethane (5 mL) and the combined organic layers were dried over MgSO4. Column chromatography of the residue (dichloromethane/methanol 4:1) afforded 96 mg (84%) of naloxone as a white solid mp: 181-182° C. (ethyl acetate), [lit. mp 173-175]xix [lit. 179.5° C. (toluene)]xx identical in all respects to the material described in the literature.xxi
References:
Brock University US2012/283443, 2012, A1 Location in patent:Page/Page column 18

1449113-77-2
0 suppliers
inquiry

465-65-6
62 suppliers
$27.89/004-1ml
646032-89-5
6 suppliers
$650.00/25 mg

465-65-6
62 suppliers
$27.89/004-1ml
![6,11b-Ethano-7H-furo[2',3',4',5':4,5]phenanthro[9,8a-d]oxazol-11(11aH)-one, 2-(acetyloxy)-5,5a,9,10-tetrahydro-, (5aR,6R,8aS,11aR,11bS)-](/CAS/20211123/GIF/1407592-43-1.gif)
1407592-43-1
0 suppliers
inquiry
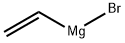
1826-67-1
211 suppliers
$51.29/100ml

465-65-6
62 suppliers
$27.89/004-1ml

1352085-46-1
31 suppliers
inquiry

465-65-6
62 suppliers
$27.89/004-1ml