
Naphazoline nitrate synthesis
- Product Name:Naphazoline nitrate
- CAS Number:5144-52-5
- Molecular formula:C14H15N3O3
- Molecular Weight:273.29

835-31-4
104 suppliers
inquiry

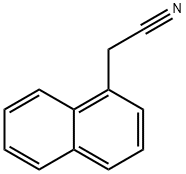
5144-52-5
201 suppliers
$35.20/25g
Yield:5144-52-5 350 mg
Reaction Conditions:
with HNO3 in lithium hydroxide monohydrate at 5 - 10; pH=2.5;
Steps:
1-3 Synthesis of naphazoline nitrate
1-naphthaleneacetonitrile (334g, 2.0mol, 1.0eq) was weighed and added to a 1L four-necked flask, then the catalyst 3-mercaptopropionic acid (6.6g) was added, and then ethylenediamine (144g) was added dropwise at a temperature of 40°C. , 2.4mol, 1.2eq), added dropwise for 1h, dripping was completed, the reaction was incubated for 8h, TLC monitored the disappearance of raw materials (VPE/EA=5:1), stopped the reaction, cooled to room temperature, a large amount of solid was precipitated, and then 95% ethanol was added (900 mL), stirring and dissolving the crude product 4,5-dihydro-2-(1-naphthylmethyl)-1H-imidazole, cooling the reaction solution to 5-10°C, then adding 65% concentrated nitric acid dropwise, adjusting pH=2.5, Until a large amount of pale yellow solid is precipitated, filtration to obtain 427 g of crude naphazoline nitrate, the yield is 78.2%, and the HPLC purity is 96.3%.Refinement of naphazoline nitrateAdd 427g of crude naphazoline nitrate to 850mL of 90% ethanol, heat to dissolve completely, then add pharmaceutical grade activated carbon (8.5g), reflux at 7580 for 30min, filter, cool to 05, filter, 50 of vacuum drying for 8 hours to obtain 350 g of white powdery solid naphazoline nitrate refined product with a yield of 91%. Measurement m.p: 167.5-170°C, purity 99.6%.
References:
CN113912545,2022,A Location in patent:Paragraph 0012; 0024-0038
132-75-2
285 suppliers
$6.00/5g

5144-52-5
201 suppliers
$35.20/25g