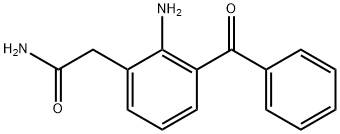
Nepafenac synthesis
- Product Name:Nepafenac
- CAS Number:78281-72-8
- Molecular formula:C15H14N2O2
- Molecular Weight:254.28
78281-61-5
81 suppliers
$165.00/250mg

78281-72-8
347 suppliers
$18.00/10mg
Yield:78281-72-8 86%
Reaction Conditions:
nickel in tetrahydrofuran;water at 20; for 0.166667 h;Product distribution / selectivity;
Steps:
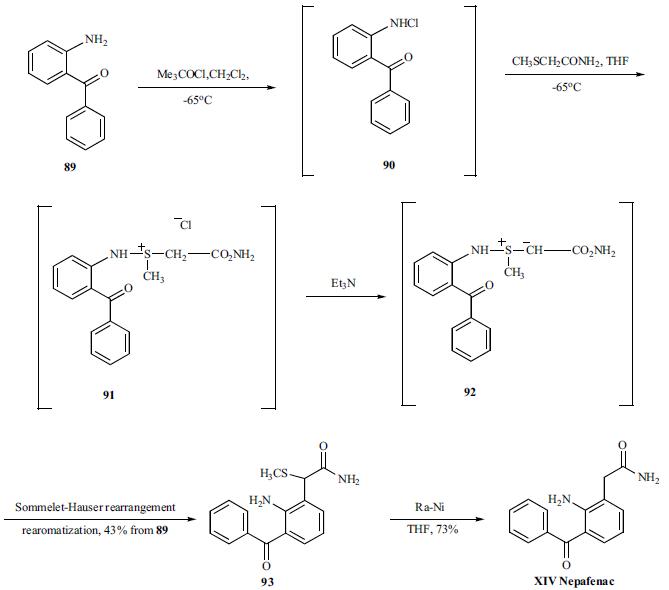
11 Preparation of 2-amino-3-benzoylbenzeneacetamide
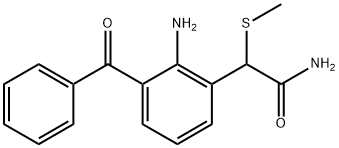
Example 11Preparation of 2-amino-3-benzoylbenzeneacetamide (i.e. nepafenac, compound of formula I).This example illustrates the use of the compound (IV) Form B obtained according to the process of the invention, for preparing nepafenac.100 g of wet Raney-Ni catalyst was loaded in the filter reactor and purged with nitrogen or argon. The catalyst was washed twice with water and once with THF (the washings were done opening the bottom valve and applying argon or nitrogen pressure in the reactor). Then 25.0 g of 2-amino-3-benzoyl-α-(methylthio)benzeneacetamide (83.2 mmol; obtained in Example 3) were dissolved in a mixture of 330 mL of THF and 80 mL of water and the resulting solution was added in one portion through the addition funnel. The reaction mixture was vigorously stirred at room temperature for 10 min. The yellow solution was unloaded from the vessel and the catalyst was washed twice with 250 mL of THF. The THF solutions were combined and distilled until a volume of 250 mL. Then, 200 mL of 2-propanol were charged twice and distilled until a remaining volume of 250 mL. The solution was allowed to cool crystallizing a yellow solid which was collected by filtration. The product was dried yielding 18.2 g of yellow crystals in needle-like shape (Yield: 86%).Analytical data: HPLC purity: 99.6% before the purification, 99.8% after the purification; No chlorination by-products were observed by HPLC; XRD: See FIG. 5; IR: See FIG. 6.
References:
Medichem, S.A. US2009/312575, 2009, A1 Location in patent:Page/Page column 5-6
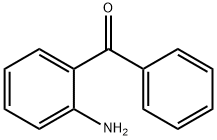
2835-77-0
425 suppliers
$8.00/5g

78281-72-8
347 suppliers
$18.00/10mg

173262-94-7
4 suppliers
inquiry

78281-72-8
347 suppliers
$18.00/10mg