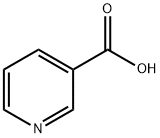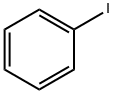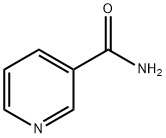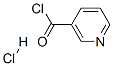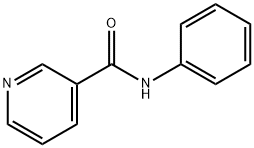
NICOTINANILIDE synthesis
- Product Name:NICOTINANILIDE
- CAS Number:1752-96-1
- Molecular formula:C12H10N2O
- Molecular Weight:198.22
Yield: 88%
Reaction Conditions:
with silica gel in neat (no solvent) at 120; for 15 h;Inert atmosphere;Sealed tube;
Steps:
14 Conversion of acids to amides
General procedure: A stirred mixture of acid 1 (1.00 mmol), amine 2 (1.05 mmol) and OSU-6 (20 wt% relative to 1) in a screw-top pressure tube (Chemglass No CG-1880-01) was placed in a pre-heated oil bath at 120 °C for the specified time (Table 2). Note: Volatile amines were used in slightly larger excess (1.10 mmol). The crude reaction mixture was dissolved with EtOAc (25 mL) and filtered. The organic layer was washed with 1M HCl (25 mL), 5% NaHCO3 (50 mL) and saturated NaCl (50 mL), and then dried (MgSO4), filtered, and concentrated under vacuum to afford the product. For the nicotinamide derivatives 3dk-3dm, the organic layer was washed with saturated NH4Cl (50 mL) rather than 1M HCl. In all cases, the products were solids and were purified by recrystallization from EtOH to afford the isolated yields shown in Table 2. To obtain analytical samples, the crude products were purified on 15cm×2.5cm silica gel columns eluted with 40-60% EtOAc in hexanes.
References:
Nammalwar, Baskar;Muddala, Nagendra Prasad;Watts, Field M.;Bunce, Richard A. [Tetrahedron,2015,vol. 71,# 48,p. 9101 - 9111]
626-60-8
360 suppliers
$13.00/5g
201230-82-2
1 suppliers
inquiry
62-53-3
631 suppliers
$10.00/1g

1752-96-1
85 suppliers
$19.00/1g

1120-90-7
297 suppliers
$5.00/250mg
201230-82-2
1 suppliers
inquiry

62-53-3
631 suppliers
$10.00/1g

1752-96-1
85 suppliers
$19.00/1g