Orcinol synthesis
- Product Name:Orcinol
- CAS Number:504-15-4
- Molecular formula:C7H8O2
- Molecular Weight:124.14
Orcinol was first prepared by dehydroacetic acid, a conversion that involved ring-opening of the pyrone to a triketone. This early experiment helped establish the rich condensation chemistry of polyketides. It can be obtained by fusing extract of aloes with potash, followed by acidification.
US3865884A: Preparation of orcinol
![Benzene, 1-methyl-3,5-bis[(trimethylsilyl)oxy]-](/CAS/20200401/GIF/89267-67-4.gif)
89267-67-4
0 suppliers
inquiry

504-15-4
374 suppliers
$7.00/100mg
Yield:504-15-4 99%
Reaction Conditions:
with methanol;1,3-disulfonic acid imidazolium hydrogen sulfate at 20; for 0.1 h;Green chemistry;
Steps:
General procedure for the deprotection of trimethylsilyl ethers
General procedure: A mixture of the substrate (1 mmol), ionic liquid [Dsim]HSO4 (6.5 mg, ∼0.02 mmol) in methanol (2 mL) was stirred at room temperature. After completion of the reaction (monitored by TLC), solvent was evaporated, water (1 mL) was added to the mixture, and stirred vigorously. Decantation of the mixture gave almost pure product(s). The products were characterized by comparison of their IR and NMR data. The ionic liquid was dried at 65 ◦C under vacuum to remove moisture, and then reused.
References:
Shirini, Farhad;Khaligh, Nader Ghaffari;Akbari-Dadamahaleh, Somayeh [Journal of Molecular Catalysis A: Chemical,2012,vol. 365,p. 15 - 23]

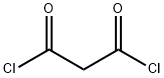
4341-24-6
176 suppliers
$8.00/1g

504-15-4
374 suppliers
$7.00/100mg

76619-89-1
86 suppliers
$34.00/100mg

4179-19-5
146 suppliers
$6.00/1g

504-15-4
374 suppliers
$7.00/100mg
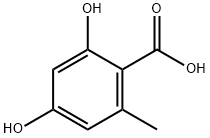
480-64-8
127 suppliers
$44.00/10mg

504-15-4
374 suppliers
$7.00/100mg