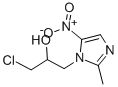
Ornidazole synthesis
- Product Name:Ornidazole
- CAS Number:16773-42-5
- Molecular formula:C7H10ClN3O3
- Molecular Weight:219.63
Yield:16773-42-5 85.2%
Reaction Conditions:
with 1-[3,5-bis(trifluoromethyl)phenyl]-3-[2-(dimethylamino)cyclohexyl]thiourea in ethyl acetate at 25 - 30; for 6 h;Solvent;Temperature;Reagent/catalyst;
Steps:
1-2 Example 1
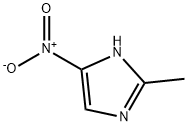
Add 127.1 g of 2-methyl-5-nitroimidazole to the reaction flask,635.5 g ethyl acetate, 4.13 g catalyst, stirMix evenly, the stirring speed is 50~300rpm,Add 127.1 g of epichlorohydrin to the above reaction flask,Control the temperature in the reaction flask to 25~30°C, stir the reaction for 6 hours, and stir at 50~300rpm;After the reaction is over, add 1 times the weight of 2-methyl-5-nitroimidazole (127.1g) of water, and adjust with 1 mol/L hydrochloric acid.Adjust the pH to about 4.0, stand for layering to obtain an organic phase and an aqueous phase;Adjust the pH to about 8.0 with 1.25 mol/L sodium hydroxide solution in the aqueous phase, and solids will precipitate out. Filter with suction. Use a small amount of solid phaseAfter rinsing with water, the catalyst is dried and can be used directlyThe organic phase was washed with water 1 times the weight of 2-methyl-5-nitroimidazole, separated into layers, and dried over anhydrous sodium sulfate.After filtering, concentrating the solvent under reduced pressure, recrystallizing with ethanol and drying to obtain the ornidazole product. Detected by HPLC, the results are shown in Figure 1, Figure 1 is the HPLC chart of ornidazole prepared in Example 1 of the present invention; from Figure 1It can be seen that the purity of the product is 99.67%; the yield is 85.2%. The following table is the retention time analysis result of Figure 1.
References:
CN111471017,2020,A Location in patent:Paragraph 0035-0040; 0047-0050