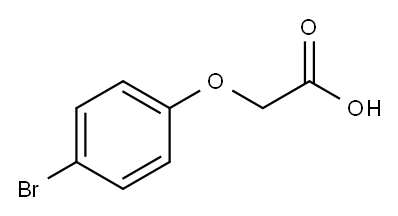
p-Bromophenoxyacetic acid synthesis
- Product Name:p-Bromophenoxyacetic acid
- CAS Number:1878-91-7
- Molecular formula:C8H7BrO3
- Molecular Weight:231.04
Yield:1878-91-7 95%
Reaction Conditions:
with sodium hydroxide in waterReflux;
Steps:
1 1.1. Synthesis of ZCL278 and its Analogs: 4-(3-(2-(4-bromo-phenoxy)-acetyl)-thioureido)-N-(4,6-dimethylpyrimidin-2-yl)-benzenesulfonamide (BA1-12) C21H20BrN5O4S2 (Bromo)
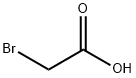
Equimolar (5-7 mmol) NaOH and substituted phenol was dissolved in 15 mL water followed by the addition of equimolar (5-7 mmol) NaOH and bromoacetic acid in 15 mL water. The solution was refluxed for 4-8 h, allowed to cool to RT, acidified with concentrated H2SO4 and extracted with ethyl acetate (3×15 mL). The organic layer was extracted with saturated NaHCO3 (3×20 mL). The aqueous layer was acidified with con. HCl followed by extraction with ethyl acetate (3×15 mL), dried over anhydrous MgSO4 and solvent removed under vacuum resulting in the substituted phenoxyacetic acid (e.g., compound 1d of Scheme 1, above). A solution of the phenoxyacetic acid in 20 mL SOCl2 and a drop of dimethyl formamide was refluxed for 3-6 h then excess SOCl2 removed by distillation to give the crude acyl chloride (e.g., compound 1e of Scheme 1, above). A solution of acyl chloride in 15 mL anhydrous acetone was added to a solution of sodium isothiocyanate in ice cold anhydrous acetone and stirred at RT for 3 h to give the acyl isothiocyanate followed by the addition of 4-amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide. After stirring overnight, the solution was poured onto ice and recrystallized in dichloromethane and methanol to give the thiourea product. 4-Bromophenol (1 g, 5.8 mmol) to afford 10 (4-bromophenoxy)acetic acid (1.27, 95%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J=11.1 Hz, 2H), 6.81 (d, J=9.1 Hz, 2H), 4.67 (s, 2H). 10 (4-bromophenoxy)acetic acid (1.5 g, 6.5 mmol) to afford the title thiourea (0.06 g, 2%) as a white 12 solid. 1H NMR (400 MHz, DMSO-d6) δ 12.30 (s, 1H), 11.76 (s, 1H), 8.04 (d, J=8.7 Hz, 2H), 7.90 (d, J=8.7 Hz, 2H), 7.53 (d, J=9.04 Hz, 2H), 7.00 (d, J=9.08 Hz, 2H), 6.81 (s, 1H), 4.95 (s, 2H), 2.31 (1, 6H). 13C NMR (DMSO-d6) δ 178.3, 169.6, 157.0, 132.2, 128.6, 123.5, 116.8, 112.7, 66.1.
References:
East Carolina University;Lu, Qun;Aguilar, Byron;Zhu, Yi;Chen, Yan-Hua US2019/91184, 2019, A1 Location in patent:Paragraph 0272; 0275

6964-29-0
28 suppliers
$40.00/5 g

1878-91-7
201 suppliers
$9.72/5g

106-41-2
482 suppliers
$13.00/5g

3926-62-3
258 suppliers
$16.69/250g

1878-91-7
201 suppliers
$9.72/5g

122-59-8
428 suppliers
$5.00/25g

1878-91-7
201 suppliers
$9.72/5g