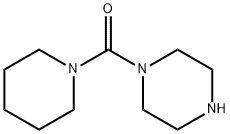
PIPERAZIN-1-YL-PIPERIDIN-1-YL-METHANONE synthesis
- Product Name:PIPERAZIN-1-YL-PIPERIDIN-1-YL-METHANONE
- CAS Number:41340-88-9
- Molecular formula:C10H19N3O
- Molecular Weight:197.28
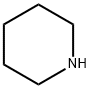
110-89-4
3 suppliers
$15.96/100ML
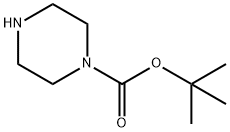
57260-71-6
737 suppliers
$5.00/5g

41340-88-9
32 suppliers
$87.00/1g
Yield:41340-88-9 36%
Reaction Conditions:
Stage #1: piperidinewith bis(trichloromethyl) carbonate;N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 3; for 1 h;
Stage #2: 1-t-Butoxycarbonylpiperazine in dichloromethane at 0 - 20;
Stage #3: with trifluoroacetic acid in dichloromethane at 20;
Steps:
10.1
Piperidine (1g, 11.752mmol) was dissolved in 20ml of dichloromethane was added N at 0 ° C ice bath, diisopropyl of N- Amine (2ml, 11.752mmol), dissolved in dichloromethane (10ml) of triphosgene (1.1g, 3.761mmol) Was slowly added dropwise, the dropping temperature was maintained at 0 ± 3 ° C, for 1 hour at 0 ° C was added an ice bath N-Boc piperazine (2.2g, 11.752mmol) And N, N- diisopropylethylamine (2ml, 11.752mmol), the reaction mixture was stirred at room temperature until TLC monitoring original Expected completion of the reaction, concentrated under reduced pressure and the crude product was dissolved in dichloromethane (30ml) was added trifluoroacetic acid (20ml, 0.27mol) The reaction was stirred at room temperature until the starting material monitored by TLC the reaction was complete, concentrated under reduced pressure, a saturated sodium carbonate solution (50ml * 3) Washed with dichloromethane (50ml * 5) was extracted, concentrated under reduced pressure and purified by silica gel column chromatography resulting residue was obtained: Piperidinyl piperazinyl urea (1.25g, pale yellow solid), yield: 36%.
References:
CN103087077,2016,B Location in patent:Paragraph 0160-0162