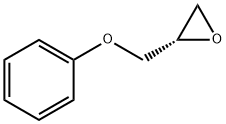
(S)-2-Oxiranylanisole synthesis
- Product Name:(S)-2-Oxiranylanisole
- CAS Number:71031-03-3
- Molecular formula:C9H10O2
- Molecular Weight:150.17
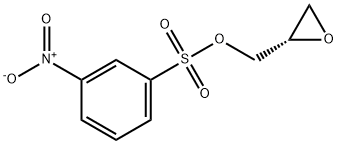
115314-14-2
251 suppliers
$5.00/250mg
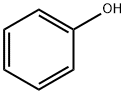
108-95-2
718 suppliers
$14.00/25g

71031-03-3
112 suppliers
$21.00/1g
Yield: 95%
Reaction Conditions:
Stage #1:phenol with sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 0.333333 h;
Stage #2:(2s)-(+)-glycidyl 3-nitrobenzenesulfonate in N,N-dimethyl-formamide at 0 - 20; for 16 h;
Steps:
1
Synthesis of (S)-2-Phenoxymethyl-oxirane (3a); To a stirred suspension of sodium hydride (60 % dispersion in mineral oil, 126 mg, 3.2 mmol) in Λ/,Λ/-dimethylformamide (4 mL) under N2 at 0 0C was added portionwise a solution of phenol (1a, 282 mg, 3.0 mmol) in N, N- dimethylformamide (1 mL) and the reaction mixture was stirred at ambient temperature for 20 min. A solution of (2S)-glycidyl m-nitrobenzenesulfonate (2, 722 mg, 2.78 mmol) in Λ/,Λ/-dimethylformamide (2 mL) was then added at 0 0C. The reaction mixture was stirred at ambient temperature for 16 h, poured onto a mixture of ice-water (15 mL) and saturated aqueous ammonium chloride solution (15 mL) and extracted with te/t-butylmethyl ether (3 * 15 mL). The combined organic layers were washed with aqueous 1Λ/ sodium hydroxide solution (2 * 30 mL), 50 % aqueous saturated brine (2 x 30 mL), and saturated brine (30 ml), dried (Na2SO4) and concentrated under reduced pressure to give (S)-2-phenoxymethyl-oxirane (3a) as a light yellow viscous oil (440 mg, 95 % yield, >95 % pure by LC-MS and 1H-NMR).
References:
Location in patent:Page/Page column 26

67843-74-7
477 suppliers
$10.00/1g

108-95-2
718 suppliers
$14.00/25g

71031-03-3
112 suppliers
$21.00/1g
70987-78-9
206 suppliers
$12.50/1g

108-95-2
718 suppliers
$14.00/25g

71031-03-3
112 suppliers
$21.00/1g

140630-45-1
0 suppliers
inquiry

71031-03-3
112 suppliers
$21.00/1g

124-38-9
122 suppliers
$175.00/23402

122-60-1
206 suppliers
$14.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

71031-03-3
112 suppliers
$21.00/1g