
TERT-BUTYL N-(2-HYDROXYETHYL)CARBAMATE synthesis
- Product Name:TERT-BUTYL N-(2-HYDROXYETHYL)CARBAMATE
- CAS Number:26690-80-2
- Molecular formula:C7H15NO3
- Molecular Weight:161.2
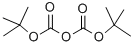
24424-99-5
816 suppliers
$13.50/25G

141-43-5
857 suppliers
$9.00/10g

26690-80-2
327 suppliers
$6.00/5g
Yield:26690-80-2 100%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 0; for 2 h;
Steps:
8 Synthesis of compounds [compounds represented by formula (4)]:
Ethanolamine (500 mg, 8.2 mmol) and sodium hydroxide (1.7 g, 40 mmol) were suspended in a mixed solvent of dioxane and water (10:1, 50 ml), and di-tert-butyl dicarbonate (1.96 g, 9.0 mmol) was added to the thus obtained suspension in several portions at 0°C. The reaction solution was stirred at 0°C for 2 hours, and then an aqueous potassium hydrogensulfate solution (5%, 50 ml) was added thereto, followed by extraction with ethyl acetate (150 ml). The extracted organic layer was washed with water (30 ml) and a saturated brine (30 ml) in this order, dried over anhydrous magnesium sulfate and then filtered. The solvent was evaporated under reduced pressure to give crude (2-hydroxyethyl)carbamic acid tert-butyl ester (1.32 g, 8.2 mmol, yield 100%). A toluene solution of Mitsunobu reagent (diethyl azodicarboxylate) (1.106 g/ml, 8.2 mmol, 1.29 ml) was gradually added to a tetrahydrofuran solution (25 ml) of triphenylphosphine (2.15 g, 8.2 mmol) at -78°C. To the thus obtained solution, a tetrahydrofuran solution (5 ml) of (2-hydroxyethyl)carbamic acid tert-butyl ester (1.32 g, 8.2 mmol) and maleimide (794 mg, 8.2 mmol) was added, followed by stirring at -78°C for 5 minutes and further stirring at room temperature for 36 hours. The thus obtained reaction solution was concentrated, ether was added thereto, and the formed precipitate was removed by filtration. The thus obtained filtrate was concentrated, and the resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1) to give 2-(maleimido)ethylcarbamic acid tert-butyl ester (531 mg, 2.53 mmol, yield 31%).1H-NMR (CDCl3, 400MHz) δ (ppm): 6.72 (2H, s), 4.75 (1H, br, NH), 3.66 (2H, t, J=5.6Hz), 3.33 (2H, dt, J=5.1, 5.6 Hz), 1.40 (9H, s). (2-Maleimido)carbamic acid tert-butyl ester (48 mg, 0.2 mmol) was dissolved in a mixed solvent of trifluoroacetic acid and dichloromethane (30:70, 5 ml), followed by stirring at 0°C for 2 hours, and then the reaction solution was concentrated. The thus obtained residue was dissolved in dichloromethane (20 ml) and a saturated aqueous sodium hydrogencarbonate solution (10 ml) was added thereto to separate the layers. The organic layer was dried over potassium hydrogencarbonate and filtered. The solvent was evaporated under reduced pressure to give 1-(2-aminoethyl)-3-pyrroline-2,5-dione (28 mg, 0.2 mmol, yield 100%). Compound 7 (77.2 mg, 0.1 mmol) and 1-(2-aminoethyl)-3-pyrroline-2,5-dione (28.0 mg, 0.2 mmol) were suspended in water (5 ml), followed by stirring at room temperature for 2 hours. The reaction solution was concentrated, and the thus obtained residue was purified by preparative reverse phase tin layer chromatography (preparative reverse phase TLC) (water:methanol = 10:1) to give Compound 33 (39 mg, 0.05 mmol, yield 50%).1H-NMR (CD3OD, 300MHz) δ (ppm): 6.56 (2H, s), 4.22-4.05 (6H, m), 3.80-3.43 (32H, m), 2.96-2.74 (4H, m).
References:
KYOWA HAKKO KOGYO CO., LTD.;Tecno Network Shikoku Co.,Ltd. EP1550651, 2005, A1 Location in patent:Page/Page column 32-33

24424-99-5
816 suppliers
$13.50/25G

2002-24-6
193 suppliers
$5.00/100mg

26690-80-2
327 suppliers
$6.00/5g

203738-69-6
18 suppliers
$505.99/5MG

26690-80-2
327 suppliers
$6.00/5g

