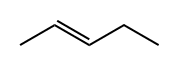
trans-2-Pentene synthesis
- Product Name:trans-2-Pentene
- CAS Number:646-04-8
- Molecular formula:C5H10
- Molecular Weight:70.13

109-66-0
420 suppliers
$10.00/25ml
504-60-9
88 suppliers
$180.00/1ml

627-20-3
50 suppliers
$42.70/1g

646-04-8
64 suppliers
$45.00/500mg

109-67-1
162 suppliers
$35.00/25 g
Yield:-
Reaction Conditions:
with ethene;Ir(2,6-bis[di(i-propyl)phosphinomethyl]phenyl)(C2H4) at 240; under 1520.1 Torr; for 1.66667 h;Glovebox;Reagent/catalyst;Time;
Steps:
2 Dehydrogenation of n-pentane with ethylene
In a typical experimental set up, a 100 tl stock solution of n-pentane containing 1 mM iridium pincer complex catalyst was taken in a few custom made thick walled 1.5 ml vials inside a glove box. The vials were then connected to Kontes adapter via tygon tubings and degassed in a high vacuum line. One atmosphere of propene was then introduced to the system and the kontes valves were sealed. The contents of the vials were frozen in liquid nitrogen and the vials were flame sealed. Note that one atm propene charged in a 3 ml space condenses to a 1.5 ml vial space upon flame sealing rendering the amount of propene in each vial to be about 2 atm. The vials were then placed in a preheated aluminum block inside a gas chromatography (GC) oven maintained at 240 °C and subjected to interval free heating for a stipulated time. The GC ovenwas then cooled to room temperature, the vials were taken out, the contents were frozen in liquid nitrogen and the tubes were broken open. The contents of each vial were analyzed by GC. Table 2. Dehydrogenation of n-pentane catalyzed by pincer iridium complexes at ethylene pressure of 2 atm (“1.2 M”) at 240°C Reaction conditions: 1mM pincer iridium catalysts in neat pentane[8.7 M] under 2 atm ethylene ‘[12M]’ at 240 °C
References:
CHEVRON U.S.A. INC.;RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY;KUMAR, Akshai;MIRONOV, Oleg;SAXTON, Robert, J.;GOLDMAN, Alan, Stuart WO2015/134663, 2015, A1 Location in patent:Paragraph 0074; 0084

627-19-0
109 suppliers
$10.00/1g

627-20-3
50 suppliers
$42.70/1g

646-04-8
64 suppliers
$45.00/500mg

109-67-1
162 suppliers
$35.00/25 g

109-66-0
420 suppliers
$10.00/25ml

627-21-4
75 suppliers
$41.00/5mL

627-20-3
50 suppliers
$42.70/1g

646-04-8
64 suppliers
$45.00/500mg

109-66-0
420 suppliers
$10.00/25ml

109-66-0
420 suppliers
$10.00/25ml

646-04-8
64 suppliers
$45.00/500mg

109-67-1
162 suppliers
$35.00/25 g

627-21-4
75 suppliers
$41.00/5mL

646-04-8
64 suppliers
$45.00/500mg