
TRICAPRIN synthesis
- Product Name:TRICAPRIN
- CAS Number:621-71-6
- Molecular formula:C33H62O6
- Molecular Weight:554.84
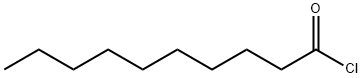
112-13-0
269 suppliers
$14.00/25g
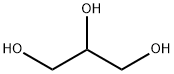
56-81-5
1623 suppliers
$5.00/25g

621-71-6
148 suppliers
$13.00/1g
628-13-7
411 suppliers
$9.00/25g
Yield:621-71-6 86%
Reaction Conditions:
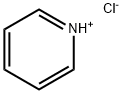
Stage #1:n-decanoyl chloride;glycerol with pyridine in dichloromethane at 20 - 35; for 0.0833333 - 0.25 h;
Stage #2: with dmap at 20;
Steps:
1
Comparative lipid 1: synthesis TRICAPRIN (Glycerol tridecanoate) Small Scale Glycerol (3.0 g, 0.0325 mol, 1 eq) pyridine (8.1 ml, 0.10 mol, 3.1 EQ) and dichloromethane (100 ml) were stirred at room temperature under nitrogen. Decanoyl chloride (21 ml, 19.25 g, 0.10 mol, 3.1 equiv) was then added dropwise over 5 min, with external cooling in a water bath to keep the temperature at 30-35 °C. When the addition was complete 4-dimethylaminopyridine (DMAP (0.12 g, 1 mmol, 0.03 eq) was added and the mixture stirred under nitrogen at room temperature overnight. The precipitated pyridine hydrochloride was removed by filtration and washed with dichloromethane. The combined washing and filtrate was then washed with aqueous solutions (20 ml) of 5% sodium chloride, 5% sodium bicarbonate, O. 1N hydrochloric acid, and 5% sodium chloride. The dichloromethane layer was then dried over MGS04 and the solvent removed III vacuo. The residual oil crystallised on standing. This material was recrystallised from isopropanol (40 ML) to give 15.6 g (86% yield) of a waxy white solid. ANALVSIS GC-99.8% pure HPLC (C18 4.6 x 100 mm, ACN/THF 85/15 1 ml/min, K 210 NM)-94. 9% pure Large Scale The above was repeated on 15 times the scale. Glycerol (45.0 g, 0.49 mol, 1 eq), pyridine (121.5 ml, 1.50 mol, 3.1 EQ) and dichloromethane (1.5 L) were stirred at room temperature under nitrogen. Decanoyl chloride (315 ml, 288.8 g, 1.50 mol, 3.1 equiv) was then added dropwise over 15 min, with external cooling in a water bath to keep the temperature at 30-35 °C. When the addition was complete 4-dimethylaminopyridine (DMAP (1.8 g, 15 mmol, 0.03 EQ) was added and the mixture stirred under nitrogen at room temperature overnight. The precipitated pyridine hydrochloride was removed by filtration and washed with dichloromethane. The combined washing and filtrate was then washed with aqueous solutions (300 ml) of 5% sodium chloride, 5% sodium bicarbonate, O. 1N hydrochloric acid, and 5% sodium chloride. The dichloromethane layer was then dried over MGS04 and the solvent removed I72 VACUO. The residual oil crystallised on standing. This material was recrystallised from isopropanol (400 ml) to give 228 g (86% yield) of a waxy white solid. ANALVSIS GC-99.8% pure HPLC (C18 4.6 x 100 mm, ACN/THF 85/15 1 ml/min, X 210 nm) -94. 9% pure A further batch was made and combined with the small-scale batch above and recrystallised from isopropanol to give 44 g of product. The above batches were combined (268 g) and reanalysed: GC 99.9% pure HPLC 97.9% Summary 263 g of glycerol tridecanoate (tricaprin, CCC) was been prepared from decanoyl chloride (98 %) by a one-step process (scheme given below). It is a white, low-melting solid and was stored under nitrogen in the freezer. The C content was 99.9 % of fatty acid content and the HPLC purity was 97.9 %.
References:
BTG INTERNATIONAL LIMITED WO2005/18632, 2005, A1 Location in patent:Page/Page column 26-27
334-48-5
586 suppliers
$6.00/25g

56-81-5
1623 suppliers
$5.00/25g

621-71-6
148 suppliers
$13.00/1g
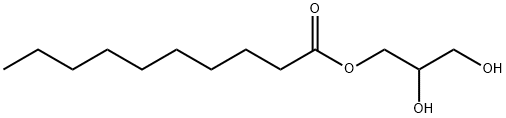
2277-23-8
51 suppliers
$7.00/250mg

17863-69-3
5 suppliers
inquiry
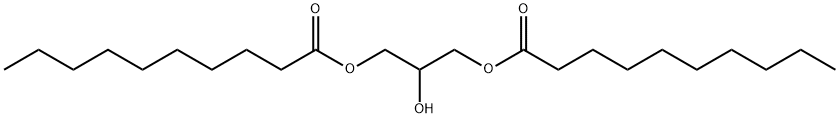
17598-93-5
25 suppliers
$25.00/1mg

334-48-5
586 suppliers
$6.00/25g

56-81-5
1623 suppliers
$5.00/25g

621-71-6
148 suppliers
$13.00/1g

2277-23-8
51 suppliers
$7.00/250mg

17598-93-5
25 suppliers
$25.00/1mg