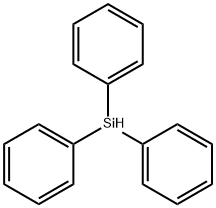
Triphenylsilane synthesis
- Product Name:Triphenylsilane
- CAS Number:789-25-3
- Molecular formula:C18H16Si
- Molecular Weight:260.41
3 PhSiH3 + 2 LiAlH4 → C18H16Si + 2 LiH + 2 AlH3
This reaction is typically carried out in anhydrous conditions and under inert gas atmosphere.
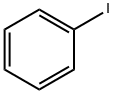
76-86-8
204 suppliers
$16.00/5g

789-25-3
204 suppliers
$6.00/1g
Yield:789-25-3 65%
Reaction Conditions:
with sodium tetrahydroborate in acetonitrile at 20; for 0.25 h;Inert atmosphere;
Steps:
2.2-2 Synthesis of example 2
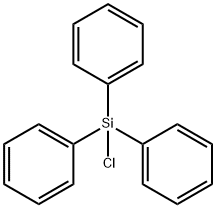
After replacing the interior of a 7 mL test tube equipped with a stirring bar with nitrogen gas,Under nitrogen flow,In this test tube,0.5 mL of acetonitrile as a reaction solvent,As a reducing agent, sodium borohydride (NaBH 4, ReagentPlus (registered trademark), manufactured by Sigma-Aldrich Japan Co.,Purity 99%) 37.8 mg (1.0 mmol)To prepare a suspension.To this suspension,147.4 mg (0.5 mmol) of triphenylchlorosilane (Ph 3 SiCl) was added,The reaction was allowed to proceed for 15 minutes while stirring at room temperature.After the reaction,Acetonitrile was distilled off,To the obtained product (mixture), 5 mL of n-hexane was added under the atmosphere, and the mixture was filtered using a silica pad,By drying,85.0 mg (0.326 mmol) of triphenylsilane (Ph 3 SiH) was isolated.The yield of triphenylsilane was 65%
References:
NIPPON SHOKUBAI COMPANY LIMITED;OSAKA CITY UNIVERSITY;ABE, TAKASHI;NAKAZAWA, HIROSHI JP2017/160160, 2017, A Location in patent:Paragraph 0039; 0040

10025-78-2
168 suppliers
$22.50/25g

789-25-3
204 suppliers
$6.00/1g

16343-18-3
40 suppliers
$60.00/50mg
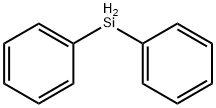
775-12-2
200 suppliers
$10.00/1g

789-25-3
204 suppliers
$6.00/1g
694-53-1
227 suppliers
$17.46/1G

775-12-2
200 suppliers
$10.00/1g

7440-21-3
204 suppliers
$30.00/100 gm

789-25-3
204 suppliers
$6.00/1g