Vincamine synthesis
- Product Name:Vincamine
- CAS Number:1617-90-9
- Molecular formula:C21H26N2O3
- Molecular Weight:354.44
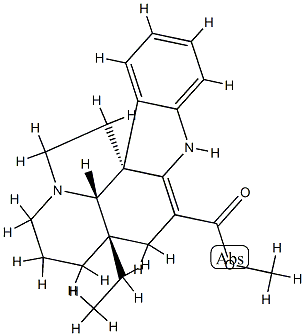
3247-10-7
1 suppliers
inquiry

1617-90-9
395 suppliers
$6.00/25mg
Yield:1617-90-9 78.3%
Reaction Conditions:
Stage #1:(-)-vincadifformine with monoperoxymaleic acid in methanol at -5 - 0; for 6.16667 h;
Stage #2: with sodium dithionate at 40; for 3 h;Reagent/catalyst;
Steps:
4.4 (4) Preparation of vincamine
To a 1000 mL four-necked flask, 24.0 g (70.9 mmol)Changchun Buddha Ming,133.0 g of methanol was dissolved,Cool to -5-0 ,With stirring, a solution of 119.0 g (60.9 mmol)Monoperoxymaleic acid,Stirring 40min after the completion of the oxidation reaction,119.1 g (60.9 mmol) of monoperoxymaleic acid was further added dropwise,2.5h drop end,Continue stirring 3h oxidation reaction.After the reaction is completed,Dropping 40% concentration of reducing agent solution to starch KI test strip no longer discolored,Continue stirring 10min,Heated to 40 3h stirring rearrangement reaction,The reductant selection is shown in Table 4.After the reaction is completed,433.9 g of water,Cool to 25 ,Dropping ammonia solution to adjust the pH to 8.5-9,Continue stirring to cool to 0-5 ,Crystallization 2h after filtration,The filter cake was washed with 57.0 g of cold methanol,150g water washing, drained,Filter cake was added to 500mL single-necked flask,Add 300g of water,Stirring heated to 60 beating 2h,Suction filtration, vacuum dried at 40 ° C to obtain vinblastine,Yield and quality yield are shown in Table 4.
References:
Northeast Pharmaceutical Group Co., Ltd.;Li Yang;Zhang Weijun;Liu Suna;Bai Jie;Tao Fang;Di Fan;Dong Aijun;Yang Lu;Li Ye;Wang Long;Han Xiaodan CN106749230, 2017, A Location in patent:Paragraph 0052; 0053; 0064; 0065; 0076-0079; 0088-0094

4429-63-4
187 suppliers
$35.00/1mg

1617-90-9
395 suppliers
$6.00/25mg
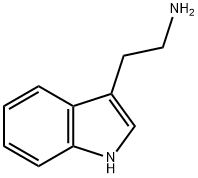
61-54-1
501 suppliers
$12.00/5 g

1617-90-9
395 suppliers
$6.00/25mg
![ethyl 3-{[2-(1H-indol-3-yl)ethyl]amino}propanoate](/CAS/20180601/GIF/14487-98-0.gif)
14487-98-0
4 suppliers
inquiry

1617-90-9
395 suppliers
$6.00/25mg