| Identification | More | [Name]
N-((4-Aminophenyl)sulfonyl)acetamide | [CAS]
144-80-9 | [Synonyms]
AKOS BBB/023
LABOTEST-BB LT00053160
N1-ACETYLSULFANILAMIDE
N-(4-AMINOBENZENESULFONYL)ACETAMIDE
N-(4-AMINOPHENYLSULFONYL)ACETAMIDE
N-ACETYL-4-AMINO-BENZENESULFONAMIDE
N'-ACETYLSUFANILAMIDE
N-ACETYLSULFANILAMIDE
N-[P-AMINOBENZENESULFONYL]ACETAMIDE
N-SULFANILYACETAMIDE
N-SULFANILYLACETAMIDE
p-aminobenzenesulfonacetamide
SULFACETAMIDE
sulfanilacetamide
SULPHACETAMIDE
sultrin
A-500
Acetamide, N-[(4-aminophenyl)sulfonyl]-
Acetamide, N-sulfanilyl-
Acetocid | [EINECS(EC#)]
205-640-6 | [Molecular Formula]
C8H10N2O3S | [MDL Number]
MFCD00066501 | [Molecular Weight]
214.24 | [MOL File]
144-80-9.mol |
| Chemical Properties | Back Directory | [Appearance]
white crystalline powder | [Melting point ]
182-184 °C
| [density ]
1.3844 (rough estimate) | [refractive index ]
1.5690 (estimate) | [storage temp. ]
0-6°C | [solubility ]
ethanol: soluble50mg/mL | [form ]
powder | [pka]
pKa 1.76±0.04(H2O
t = 25
I = 0.15 (KCl)
Ar atmosphere)(Approximate);5.22±0.01(H2O
t = 25
I = 0.15 (KCl)
Ar atmosphere)(Approximate) | [color ]
white to off-white | [Stability:]
Stable. Incompatible with strong oxidizing agents, strong bases, strong reducing agents, strong acids. | [Water Solubility ]
<0.01 g/100 mL at 16 ºC | [Merck ]
14,8899 | [BRN ]
981718 | [InChIKey]
SKIVFJLNDNKQPD-UHFFFAOYSA-N | [CAS DataBase Reference]
144-80-9(CAS DataBase Reference) | [NIST Chemistry Reference]
N-(p-Aminobenzenesulfonyl)acetamide(144-80-9) | [EPA Substance Registry System]
144-80-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
2
| [RTECS ]
AC8450000
| [TSCA ]
Yes | [HS Code ]
29350090 | [Safety Profile]
Mildly toxic by ingestion and intravenous routes. An experimental teratogen. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and SOx. | [Toxicity]
LD50 orally in dogs: 8000 mg/kg (Fisher) |
| Hazard Information | Back Directory | [General Description]
White powder. | [Reactivity Profile]
SULFACETAMIDE(144-80-9) is an amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generate mixed oxides of nitrogen (NOx) | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available. SULFACETAMIDE is probably combustible. | [Chemical Properties]
white crystalline powder | [Originator]
Sulamyd,Schering,US,1941 | [Uses]
Sulfacetamide is an antibiotic used for the treatment of skin infections and urinary tract infections. Sulfacetamide is also used to treat acne and seborrheic dermatitis. Sulfacetamide was investigate
d for potential anti-inflammatory properties | [Definition]
ChEBI: A sulfonamide that is sulfanilamide acylated on the sulfonamide nitrogen. | [Manufacturing Process]
17.2 grams of 4-aminobenzene-sulfonamide are heated to boiling with 75 cc
of acetic anhydride for 1 hour and thereupon the diacetyl product caused to
separate by stirring into ice water. After recrystallization from alcohol the 4-
acetylaminobenzene-sulfonacetyl-amide forms colorless prisms of melting
point 253°C with decomposition. The product is easily soluble in alkalies and
forms neutral salts. The acetylation can also take place with acetyl chloride.
Instead of the 4-aminobenzene-sulfonamide also 4-acetylaminobenzenesulfonamide
can be employed. The action of 4-acetyla
By heating the diacetyl compound with sodium hydroxide solution partial
saponification of the acetyl groups takes place. 25.6 grams of diacetyl
compound are heated to boiling for some hours with 100 cc of 2N sodium
hydroxide solution. The precipitate produced by acidification of the solution
with acetic acid is filtered off and treated with dilute sodium carbonate
solution. The 4-aminobenzene-sulfonacetylamide passes into solution while
the simultaneously formed 4-acetylaminobenzene-sulfonamide remains
undissolved. It is filtered with suction and the filtrate again acidified with
acetic acid. The 4-aminobenzene-sulfon-acetamide separates out and is
recrystallized from water. It forms colorless lustrous rhombic crystals of MP
181°C. | [Therapeutic Function]
Antimicrobial | [Pharmaceutical Applications]
N-acetylsulfanilamide. It is very soluble in water and was
formerly used in urinary tract infection. It is available in
some countries in ophthalmic preparations and as a component
(with sulfathiazole and sulfabenzamide) of a triple
sulfonamide cream for the topical treatment of bacterial
vaginosis.
Sulfacetamide is one of the least active sulfonamides. It
is well absorbed when given orally and is excreted in the
urine with a half-life of around 9 h. About 70% is excreted
unchanged, the remainder being present as the acetyl metabolite.
Adverse reactions are those common to the group.
Stevens–Johnson syndrome has been reported several times
after topical use in conjunctivitis. | [Biochem/physiol Actions]
Sulfacetamide is a sulfonamide antibiotic that blocks the synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase. Sulfacetamide is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), which is required for bacterial synthesis of folic acid. It is active against Gram positive bacteria, Gram negative bacteria and Chlamydia. Mode of resistance is via the alteration of dihydropteroate synthase or alternative pathway for folic acid synthesis. | [Synthesis]
Sulfacetamide, N1
-acetylsulfanilamide (33.1.44), is synthesized either
by direct alkylation of acetamide with 4-aminobenzenesulfonyl chloride, or by reacting
4-aminobenzenesulfonamide with acetic anhydride and subsequent selective, reductive dea�cylation of the resulting acetamide 33.1.45 using a system of zinc¨Csodium hydroxide.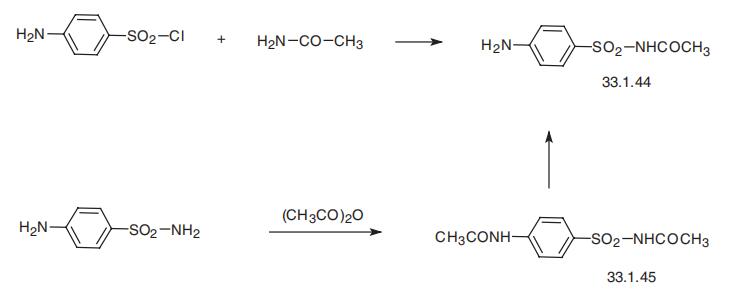
| [Purification Methods]
Crystallise the amide from aqueous EtOH. [Beilstein 14 IV 2662.] |
|
|