| Identification | More | [Name]
Bromocriptine | [CAS]
25614-03-3 | [Synonyms]
BROMOCRIPTINE
(5’-alpha)-ropyl)
,6’,18-trione
2-bromo-12’-hydroxy-2’-(1-methylethyl)-5’-alpha-(2-methylpropyl)ergotamin-3’
2-bromo-alpha-ergocryptine
2-bromo-alpha-ergokryptin
2-bromo-alpha-ergokryptine
2-bromo-ergocryptin
2-bromoergocryptine
alpha-bromoergocriptine
bromergocryptine
bromocriptin
bromocryptine
bromoergocriptine
bromoergocryptine
ergotaman-3’,6’,18-trione,2-bromo-12’-hydroxy-2’-(1-methylethyl)-5’-(2-methylp
(5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)ergotaman-3',6',18-trione
Kripton
Parlodel
Bromoergocryptine mesylate | [EINECS(EC#)]
247-128-5 | [Molecular Formula]
C32H40BrN5O5 | [MDL Number]
MFCD00870213 | [Molecular Weight]
654.59 | [MOL File]
25614-03-3.mol |
| Chemical Properties | Back Directory | [Definition]
An semisynthetic ergotamine alkaloid derivative and
powerful dopamine D2 agonist. It inhibits prolactin
secretion and release from the pituitary and retards
tumor growth.
| [Appearance]
Crystals. | [Melting point ]
215-218° (dec) | [alpha ]
D20 -195° (c = 1 in methylene chloride) | [Boiling point ]
891.3±65.0 °C(Predicted) | [density ]
1.2734 (rough estimate) | [refractive index ]
1.6400 (estimate) | [pka]
pKa 4.90±0.05(80% MCS
t = RT) (Uncertain) | [Water Solubility ]
2.07mg/L(temperature not stated) | [CAS DataBase Reference]
25614-03-3(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Hazard]
Poison; teratogen; developmental abnor-
malities of the respiratory system,musculoskeletal
system, rogenital system, craniofacial area, and
body wall; teratogen; mutagen; questionable car-
cinogen; tumorigen; causes nausea, vomiting,
orthostatic hypotension; constipation, dyskinesias,
psychoses, digital spasm, erythromelalgia.
| [Chemical Properties]
Crystals. | [Originator]
Parlodel,Sandoz,UK,1975 | [Uses]
Bromocriptine, a dopaminomimetic that is a dopamine D2 receptor agonist, possesses
expressed antiparkinsonian activity. It is used for treating all phases of idiopathic and post�encephalic Parkinsonism. | [Uses]
Enzyme inhibitor (prolactin). | [Manufacturing Process]
A solution of 3.4 grams of N-bromosuccinimide in 60 cc of absolute dioxane is
added drop wise in the dark, during the course of 5 minutes, to a stirred
solution, heated to 60°C, of 9.2 grams of ergocryptine in 180 cc of absolute
dioxane. The reaction mixture is stirred at this temperature for 70 minutes
and is concentrated to a syrup-like consistency in a rotary evaporator at a
bath temperature of 50°C. The reaction mixture is subsequently diluted with
300 cc of methylene chloride, is covered with a layer of about 200 cc of a 2 N
sodium carbonate solution in a separating funnel and is shaken thoroughly.
The aqueous phase is extracted thrice with 100 cc amounts of methylene
chloride. The combined organic phases are washed once with 50 cc of water,
are dried over sodium sulfate and the solvent is removed under a vacuum.
The resulting brown foam is chromatographed on a 50-fold quantity of
aluminum oxide of activity II-III with 0.2% ethanol in methylene chloride as
eluant, whereby the compound indicated in the heading is eluted immediately
after a secondary fraction which migrates somewhat more rapidly than the
fractions containing the heading compound. The last fractions to leave the
aluminum oxide contain varying amounts of starting material together with
the heading compound, and may be subjected directly, as mixed fractions, to
an afterbromination in accordance with the method described above. The
fractions containing the pure heading compound are combined and crystallized
from methyl ethyl ketonehopropy1 ether. Melting point 215°-218°C
(decomp.), [α]D
20-195° (c = 1 in methylene chloride). | [Brand name]
Parlodel (Novartis);Bromed;Lactismine;Parilac;Umprel. | [Therapeutic Function]
Prolactin inhibitor | [World Health Organization (WHO)]
Bromocriptine, a semisynthetic ergot alkaloid derivative and
prolactin inhibitor was introduced into medicine in 1976. It is used in the
prevention of lactation, but because of the risk of rebound effect and since only
10% of women benefit therapeutically from such intervention, the United States
Food and Drug Administration has requested manufacturers to no longer indicate
preparations containing bromocriptine for this purpose. The World Health
Organization is not aware of similar action having been taken elsewhere. | [Mechanism of action]
Bromocriptine is
absorbed after oral administration, but approximately 90% of a dose undergoes extensive
first-pass hepatic metabolism, with the remainder hydrolyzed in the liver to inactive metabolites
that are eliminated mostly in the bile. The half-life is relatively short (~3 hours). | [Clinical Use]
Bromocriptine is an ergot peptide derivative that is a partial agonist at D1-type and a
full agonist at D2-type postsynaptic dopamine receptors, usually given in combination with levodopa therapy. It was the first direct dopamine receptor
agonist used in treatment of Parkinson's disease after its development as an inhibitor of prolactin
release (via activation of anterior pituitary D2 receptors). At low doses (typically 1–5 mg/day),
bromocriptine is an effective prolactin inhibitor, and at higher doses (typically 10–20 mg/day), the
antiparkinsonism and mood-elevating effects of bromocriptine become apparent. | [Side effects]
Bromocriptine has a number of undesirable side effects, even caus�ing mental disturbances in long-term use. | [Synthesis]
Bromocriptine, 2-bromoergocriptine (10.1.13), is a semisynthetic deriva�tive of a natural ergot alkaloid, ergocriptin (a derivative of lysergic acid), which is synthe�sized by bromination of ergocriptin using N-bromosuccinimide [18,19].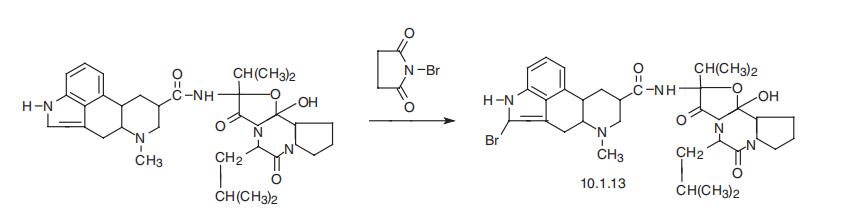
| [Drug interactions]
Potentially hazardous interactions with other drugs
Increased risk of toxicity with bromocriptine and
isometheptene. | [Metabolism]
Bromocriptine is extensively metabolised. It undergoes
extensive first-pass biotransformation in the liver,
reflected by complex metabolite profiles and by almost
complete absence of parent drug in urine and faeces.
In plasma the elimination half life is 3-4 hours for the
parent drug and 50 hours for the inactive metabolites.The parent drug and its metabolites are also completely
excreted via the liver with only 6% being eliminated via
the kidney. |
| Safety Data | Back Directory | [Toxicity]
An ergot alkaloid
derivative that exhibits potent dopamine agonist properties, particularly
at D2 dopamine receptors. Bromocriptine, like dopamine,
inhibits prolactin release from the pituitary and so is used in endocrine
disorders, such as hyperprolactinemia. It is also used in the
treatment of Parkinson’s disease. A large “first-pass” effect is seen
with bromocriptine, and peak concentrations occur about 1.5-3 h
after ingestion, with a half-life of about 3 h. Nausea, vomiting, and
orthostatic hypotension are among the acute adverse effects.
Long-term use has been associated with dyskinesias, constipation,
psychoses, digital spasm, and erythromelalgia. The LD50 in rabbits
exceeds 1 g/kg, p.o., and 12 mg/kg, i.v. |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
NCE Biomedical Co.,Ltd.
|
| Tel: |
4000-027-021 |24 +86-13986109188 | +86-15623472865 | +81-08033611988 |
| Website: |
www.chemicalbook.com/ShowSupplierProductsList15748/0.htm |
|