| Identification | More | [Name]
Isoflurane | [CAS]
26675-46-7 | [Synonyms]
1-CHLORO-2,2,2-TRIFLUOROETHYL DIFLUOROMETHYL ETHER
ISOFLURANE
1-Chloro-2,2,2-trifluoroethyl ether
1-Chloro-2,2,2-trifluoroethyldrfluoromethylether
2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethan
2-Chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane
Aerrane
chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane
chloro-2,2,2-trifluorodifluoromethylether
Compd 469
Compound 469
Ethane, 1-chloro-1-(difluoromethoxy)-2,2,2-trifluoro-
Ethane, 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-
Ether, 1-chloro-2,2,2-trifluoroethyl difluoromethyl
ether,1-chloro-2,2,2-trifluoroethyldifluoromethyl
Forane
Forene
r-e235dal
Isoflurane266
1-Chloro-2,2,2-trifluoroethyl difluoromethyl ether 99% | [EINECS(EC#)]
247-897-7 | [Molecular Formula]
C3H2ClF5O | [MDL Number]
MFCD00066609 | [Molecular Weight]
184.49 | [MOL File]
26675-46-7.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid | [Melting point ]
48.5°C | [Boiling point ]
48.5 °C | [density ]
1.45 | [vapor pressure ]
238 mmHg ( 20 °C) | [refractive index ]
1.3002 | [Fp ]
48-49°C | [storage temp. ]
2-8°C | [solubility ]
Practically insoluble in water, miscible with ethanol and trichloroethylene. | [form ]
neat | [color ]
Colorless to Almost colorless | [Specific Gravity]
approximate 1.50 | [Stability:]
Stable. | [Water Solubility ]
Soluble in chloroform and ethyl acetate. Not miscible or difficult to mix in water. | [Merck ]
14,5175 | [BRN ]
1852087 | [InChIKey]
PIWKPBJCKXDKJR-UHFFFAOYSA-N | [CAS DataBase Reference]
26675-46-7(CAS DataBase Reference) | [NIST Chemistry Reference]
1-Chloro-2,2,2-trifluoroethyl difluoromethyl ether(26675-46-7) | [EPA Substance Registry System]
26675-46-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,T | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 3334 | [WGK Germany ]
3 | [RTECS ]
KN6799000 | [Hazard Note ]
Flammable/Toxic | [HS Code ]
2909191800 | [Hazardous Substances Data]
26675-46-7(Hazardous Substances Data) | [Toxicity]
An isomer of enflurane with
similar anesthetic properties. Isoflurane has less effect on myocardial
function, leaving the cardiovascular system normally
responsive to epinephrine or hypercarbia, and it does not cause
a marked increase in seizure susceptibility. It was not widely
used because of reports that it caused increases in liver neoplasms
in mice, but this observation has been challenged and
the compound reintroduced. |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
ANTIMONY(V) CHLORIDE-->BIS(CHLOROMETHYL)ETHER-->2,2,2-Trifluoroethanol-->HYDROGEN FLUORIDE GAS-->2,2,2-TRIFLUOROETHYL METHYL ETHER-->1-CHLORO-2,5-DIMETHYL-4-NITROBENZENE2-CHLORO-5-NITRO-P-XYLENE-->hydrogen fluoride-->Hydrogen fluoride-->Methanol, difluoro- (6CI,8CI,9CI)-->DIFLUOROMETHYL 2,2,2-TRIFLUOROETHYL ETHER-->1,1-Dichloro-2,2,2-trifluoroethane-->1,1-DICHLORO-2,2,2-TRIFLUOROETHYL DIFLUOROMETHYL ETHER | [Preparation Products]
Desflurane-->2-Chloro-1,1,1,2-tetrafluoroethane |
| Hazard Information | Back Directory | [Chemical Properties]
colourless liquid | [Originator]
Forane,Ohio Medical,US,1980 | [Uses]
Isoflurane is a halogenated ether used for inhalational anesthesia. Recent studies suggest that there might be a relationship between administration of isoflurane and postoperative cognitive dysfunct
ion (POCD). | [Uses]
Solvent and dispersant for fluorinated materials. | [Definition]
ChEBI: Isoflurane is an organofluorine compound. It has a role as an inhalation anaesthetic. It is functionally related to a methoxyethane. | [Manufacturing Process]
A 1-liter 3-necked stainless steel flask was fitted with a copper "Dry Ice" cold
finger condenser, a stainless steel stirring shaft and gland and a copper gas
inlet tube. To the flask there was then added 50 g (0.23 mol) of
CF3CHClOCHCl2 and 1.5 g of SbCl5 · HF gas was then slowly bubbled through
the stirred mixture which was maintained at 0°C. The reaction was run until
0.35 mol of HCl was collected, as indicated by the titration of the effluent gas
which was dissolved in water. Following the fluorination 26 g of material were
recovered and determined to be 90% pure by vapor phase chromatography.
Fractional distillation using a 30 x 0.5 cm column packed with glass helices
gave the pure product, BP 48°C to 48.5°C. | [Brand name]
Forane (Baxter Healthcare). | [Therapeutic Function]
Inhalation anesthetic | [Biological Functions]
Isoflurane (Forane) is a structural isomer of enflurane
and produces similar pharmacological properties: some
analgesia, some neuromuscular blockade, and depressed
respiration. In contrast, however, isoflurane is considered
a particularly safe anesthetic in patients with ischemic
heart disease, since cardiac output is maintained,
the coronary arteries are dilated, and the myocardium
does not appear to be sensitized to the effects of catecholamines.
Also, blood pressure falls as a result of vasodilation,
which preserves tissue blood flow. Isoflurane
causes transient and mild tachycardia by direct sympathetic
stimulation; this is particularly important in the
management of patients with myocardial ischemia.
Unlike enflurane, isoflurane does not produce a
seizurelike EEG pattern. Furthermore, the metabolic
transformation of isoflurane is only one-tenth that of
enflurane, so fluoride production is quite low. Among
the halogenated hydrocarbons, isoflurane is one of the
most popular, since it preserves cardiovascular stability
and causes a low incidence of untoward effects. | [General Description]
Isoflurane is a volatile liquid (bp=48.5°C) with an MAC of1.15, a blood:gas partition coefficient of 1.43 and high solubilityin fat. Isoflurane is a structural isomer of enflurane. Itis a known respiratory irritant, but less so than desflurane.Approximately 0.2% of the administered drug undergoesmetabolism, the rest is exhaled unchanged. The metabolismof isoflurane yields low levels of the nephrotoxic fluoride ionas well as a potentially hepatotoxic trifluoroacetylating compound). The relatively low concentrations ofthese compounds have resulted in very low risks of hepatotoxicityand nephrotoxicity. There have been no reports ofseizures caused by isoflurane and only transient increases inheart rate have been reported. | [Biochem/physiol Actions]
Isoflurane is a tandem pore potassium channel activator. It is also a very widely used anesthetic for animal research and for in vitro studies on anesthesia mechanisms. | [Clinical Use]
Isoflurane was introduced in the United States in 1981 and is a potent anesthetic agent
with many similarities to its isomer enflurane (potent, nonflammable, and intermediate blood
solubility). It does produce significantly fewer cardiovascular effects than enflurane, however, and
it can be used safely with epinephrine without as great a concern for arrhythmia production.
Isoflurane has a more pungent odor than halothane and, thus, can cause irritation to the throat and
respiratory tract, triggering coughing and laryngospasm. To overcome this problem, it often is
supplemented with intravenous agents. Less than 0.2% of an administered dose is metabolized,
mostly to fluoride and trifluoroacetic acid. Some minimal potential
for hepatotoxicity is associated with a trifluoroacetyl halide metabolite. | [Synthesis]
Isoflurane is prepared by chlorination
of 2,2,2-trifluoroethoxydifluoromethane, itself
obtained by alkylation of trifluoroethanol with
difluorochloromethane , :
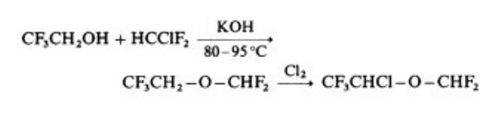 | [Veterinary Drugs and Treatments]
Isoflurane is an inhalant anesthetic that has some distinct advantages
over either halothane or methoxyflurane due to its lessened
myocardial depressant and catecholamine sensitizing effects, and
the ability to use it safely in patients with either hepatic or renal
disease. Isoflurane’s higher cost than either methoxyflurane or halothane
is a disadvantage.
Horses may recover more rapidly than with halothane, but be
more susceptible to anesthetic associated-
myopathy. |
|
|