| Identification | More | [Name]
DISOPYRAMIDE | [CAS]
3737-09-5 | [Synonyms]
ALPHA-[2-[BIS(1-METHYLETHYL)AMINO]ETHYL]-ALPHA-PHENYL-2-PYRIDINE ACETAMIDE
ALPHA-DIISOPROPYLAMINOETHYL-ALPHA-PHENYLPYRIDINE-2-ACETAMIDE
DISOPYRAMIDE
2-Pyridineacetamide, alpha-[2-(diisopropylamino)ethyl]-alpha-phenyl-
2-Pyridineacetamide, alpha-[2-[bis(1-methylethyl)amino]ethyl]-alpha-phenyl-
4-(Diisopropylamino)-2-phenyl-2-(2-pyridinyl)butanamide
4-Diisopropylamino-2-phenyl-2-(2-pyridyl)-butyramide
alpha-(2-(diisopropylamino)ethyl)-alpha-phenyl-2-pyridineacetamid
Dicorantil
gamma-Diisopropylamino-alpha-phenyl-alpha-(2-pyridyl)butyramide
H 3292
H. 3292
h3292
Isorythm
Lispine
Ritmodan
SC 7031
sc7031
Searle 703
searle703 | [EINECS(EC#)]
223-110-2 | [Molecular Formula]
C21H29N3O | [MDL Number]
MFCD00057366 | [Molecular Weight]
339.47 | [MOL File]
3737-09-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
94.5-950C | [Boiling point ]
475.43°C (rough estimate) | [density ]
1.0779 (rough estimate) | [refractive index ]
1.6300 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Soluble in DMSO (25 mg/ml) and Ethanol (>35 mg/mL) | [form ]
solid | [pka]
10.2; also reported as 10.45(at 25℃) | [color ]
White | [Water Solubility ]
6.17mg/L(22.5 ºC) | [Usage]
Antiarrhythmic (class IA). Sodium channel blocker | [Merck ]
14,3360 | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. | [CAS DataBase Reference]
3737-09-5(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
UR8432000
| [HS Code ]
2933.39.4100 | [Toxicity]
LD50 i.p. in mice: 517 mmol/kg (Ruenitz, Mokler) |
| Hazard Information | Back Directory | [Description]
Structurally, disopyramide does not belong to any of the known classes of antiarrhythmics; however, being a drug of the class IA sodium channel blockers, it exhibits membranestabilizing action and increases the effective refractory period and duration of an action potential in the atrium and ventricles. It causes a decrease in contractability and excitability of the myocardium, slowing of conductivity, and suppression of sinoatride automatism. | [Chemical Properties]
Crystalline Solid | [Originator]
Rythmodan,Cassenne,France,1969 | [Uses]
Antiarrhythmic (class IA). Sodium channel blocker | [Uses]
Antiarrhythmic;Na+ channel blocker | [Uses]
Disopyramide is used for preventing and restoring atrial and ventricular extrasystole and tachycardia in order to prevent atrial flutter and arrhythmia. | [Definition]
ChEBI: A monocarboxylic acid amide that is butanamide substituted by a diisopropylamino group at position 4, a phenyl group at position 2 and a pyridin-2-yl group at position 2. It is used as a anti-arrhythmia drug. | [Manufacturing Process]
To a solution of 35.3 parts of phenylacetonitrile and 47.6 parts of 2-
bromopyridine in 175 parts of dry toluene is added 53.4 parts of sodamide
slowly with stirring over a period of 45 minutes. The resultant mixture is
stirred at 100°C for 2 hours before it is cooled and the excess sodamide is
decomposed by the addition of water. The toluene layer is separated and
washed with water to remove excess alkali. The toluene solution is extracted
with 6 N hydrochloric acid and the acid extract is made alkaline and then
extracted with toluene. The toluene solution is dried over sodium sulfate and
the solvent is evaporated. Recrystallization of the residue from alcohol-hexane
gives α-phenyl-2-pyridineacetonitrile melting at about 87-88°C.
To a solution of 41 parts of α-phenyl-2-pyridineacetonitrile in 350 parts of dry
toluene is added 9.2 parts of sodamide and the mixture is stirred and heated
at 90°C for 30 minutes. Heating is stopped and a solution of 38.5 parts of 2-
diisopropylaminoethyl chloride in 110 parts of dry toluene is added slowly over
a period of 30 minutes. The mixture is stirred and refluxed for 6 hours before
it is cooled and decomposed by the addition of water. The toluene layer is
separated and washed with water and extracted with 6 N hydrochloric acid.
The acid extract is made alkaline and extracted with toluene. The toluene
solution is washed with water and dried and the solvent is evaporated.
Distillation of the residue gives 4-diisopropylamino-2-phenyl-2-(2-pyridyl)-
butyronitrile boiling at about 145°-160°C at 0.3 mm pressure.
A solution of 27.2 parts of 4-diisopropylamino-2-phenyl-2-(2-
pyridyl)butyronitrile in 200 parts of concentrated sulfuric acid is heated on a
steam bath for 4 hours and then poured onto ice. The resultant mixture is
alkalized with 10 N sodium hydroxide, and the pH is adjusted to 6 by the
addition of acetic acid. The solution is washed once with benzene before it is
alkalized again with 10 N sodium hydroxide solution. The resultant mixture is
extracted with benzene, and the solvent is evaporated from the benzene
extract. The resultant residue is dissolved in ethanol and the alcohol solution
is treated with charcoal and filtered. Evaporation of the solvent leaves a
residue which is recrystallized from hexane to give 4-diisopropylamino-2-
phenyl-2-(2-pyridyl)butyramide melting at about 94.5-95°C. It may be
converted to the phosphate with phosphoric acid. | [Brand name]
Norpace (Searle). | [Therapeutic Function]
Antiarrhythmic | [Mechanism of action]
Disopyramide has a pharmacological profile
similar to that of quinidine and procainamide
. Clinically, it is active against most
forms of arrhythmias (supraventricular and ventricular). | [Pharmacokinetics]
Disopyramide phosphate is used orally for the treatment of certain ventricular and atrial arrhythmias.
Despite its structural dissimilarity to procainamide, its cardiac effects are very similar.
Disopyramide is rapidly and completely absorbed from the gastrointestinal tract. Peak plasma level
is usually reached within 1 to 3 hours, and a plasma half-life of 5 to 7 hours is common.
Approximately half of an oral dose is excreted unchanged in the urine. The remaining drug
undergoes hepatic metabolism, principally to the corresponding N-dealkylated form. This metabolite
retains approximately half the antiarrhythmic activity of disopyramide and also is subject to renal
excretion. | [Clinical Use]
Disopyramide (Norpace) can suppress atrial and ventricular
arrhythmias and is longer acting than other
drugs in its class.
The indications for use of disopyramide are similar to
those for quinidine, except that it is not approved for
use in the prophylaxis of atrial flutter or atrial fibrillation
after DC conversion.The indications are as follows:
unifocal premature (ectopic) ventricular contractions,
premature (ectopic) ventricular contractions of multifocal
origin, paired premature ventricular contractions
(couplets), and episodes of ventricular tachycardia.
Persistent ventricular tachycardia is usually treated with
DC conversion. | [Side effects]
The major toxic reactions to disopyramide administration
include hypotension, congestive heart failure, and
conduction disturbances. These effects are the result of
disopyramide’s ability to depress myocardial contractility
and myocardial conduction. Although disopyramide
initially may produce ventricular tachyarrhythmias or
ventricular fibrillation in some patients, the incidence of
disopyramide-induced syncope in long-term therapy is
not known. Most other toxic reactions (e.g., dry mouth,
blurred vision, constipation) can be attributed to the anticholinergic
properties of the drug.
CNS stimulation and hallucinations are rare.The incidence
of severe adverse effects in long-term therapy
may be lower than those observed with quinidine or
procainamide. | [Synthesis]
Disopyramide, |á-(2-diisopropylaminoethyl)-|á-phenyl-2-pyridineacetamide
(18.1.6), is synthesized by arylating benzylcyanide with 2-chloropiridine in the presence of
sodium amide and subsequent alkylation of the resulting |á-phenyl-|á-(2-pyridyl) acetonitrile (18.1.4) with 2-diisopropylaminoethylchloride using sodium amide. Sulfuric acid hydrolysis
of the resulting nitrile (18.1.5) leads to the formation of |á-(2-diisopropylaminoethyl)-
|á-phenyl-2-pyridineacetamide, disopyramide.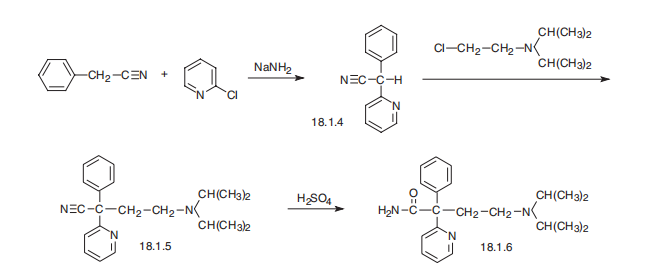
| [Drug interactions]
In the presence of phenytoin, the metabolism of disopyramide
is increased (reducing its effective concentration)
and the accumulation of its metabolites is also
increased, thereby increasing the probability of anticholinergic
adverse effects. Rifampin also stimulates the
hepatic metabolism of disopyramide, reducing its
plasma concentration.
Unlike quinidine, disopyramide does not increase
the plasma concentration of digoxin in patients receiving
a maintenance dose of the cardiac glycoside.
Hypoglycemia has been reported with the use of
disopyramide, particularly in conjunction with moderate
or excessive alcohol intake. | [storage]
Store at -20°C | [Precautions]
Disopyramide should not be administered in cardiogenic
shock, preexisting second- or third-degree A-V
block, or known hypersensitivity to the drug. Neither
should it be given to patients who are poorly compensated
or those with uncompensated heart failure or severe
hypotension. Because of its ability to slow cardiac
conduction, disopyramide is not indicated for the treatment
of digitalis-induced ventricular arrhythmias.
Patients with congenital prolongation of the QT interval
should not receive quinidine, procainamide, or disopyramide
because further prolongation of the QT interval
may increase the incidence of ventricular fibrillation.
Because of its anticholinergic properties, disopyramide
should not be used in patients with glaucoma.
Urinary retention and benign prostatic hypertrophy are
also relative contraindications to disopyramide therapy.
Patients with myasthenia gravis may have a myasthenic
crisis after disopyramide administration as a result of
the drug’s local anesthetic action at the neuromuscular
junction.The elderly patient may exhibit increased sensitivity
to the anticholinergic actions of disopyramide.
Caution is advised when disopyramide is used in
conjunction with other cardiac depressant drugs, such as verapamil, which may adversely affect atrioventricular
conduction. | [References]
1) Hell?et al.?(1978),?Disopyramide: a review of its pharmacological properties and therapeutic use in treating cardiac arrhythmias; Drugs?115?331
2) Verlinden?et al.?(2015),?Disopyramide for Hypertrophic Cardiomyopathy: A Pragmatic Reappraisal of an Old Drug; Pharmacotherapy,?35?1164
3) Nakajima?et al.?(1989),?Anti-Cholinergic Effects of Quinidine, Disopyramide, and Procainamide in Isolated Atrial Myocytes: Mediation by Different Molecular Mechanisms; Circ. Res.,?64?297 |
|
| Company Name: |
TCI Europe
|
| Tel: |
320-37350700 |
| Website: |
https://www.tcichemicals.com/de/de/index.html |
| Company Name: |
TCI AMERICA
|
| Tel: |
800-4238616 |
| Website: |
https://www.tcichemicals.com/en/us/index.html |
|