| Identification | More | [Name]
Norepinephrine | [CAS]
51-41-2 | [Synonyms]
(-)-ARTERENOL
ARTERENOL (-)-
L-ARTERENOL
LEVARTERENOL
L-NORADRENALINE
L-NOREPINEPHRINE
NORADRENALIN
(-)-NORADRENALINE
NORADRENALINE
(-)-NOREPINEPHRINE
NOREPINEPHRINE
(R)-4-(2-AMINO-1-HYDROXYETHYL)-1,2-BENZENEDIOL
(-)-alpha-(aminomethyl)protocatechuylalcohol
(-)-noradrec
4-(2-amino-1-hydroxyethyl)-2-benzenedio(r)-
adrenor
aktamin
arterenolfreebase(noradrenaline)
d-(-)-noradrenaline
l-1-(3,4-dihydroxyphenyl)-2-aminoethanol | [EINECS(EC#)]
205-750-4 | [Molecular Formula]
C8H11NO3 | [MDL Number]
MFCD00025592 | [Molecular Weight]
169.18 | [MOL File]
51-41-2.mol |
| Chemical Properties | Back Directory | [Appearance]
solid | [Melting point ]
220-230°C | [alpha ]
D25 -37.3° (c = 5 in water with 1 equiv HCl) | [Boiling point ]
298.46°C (rough estimate) | [density ]
1.2435 (rough estimate) | [refractive index ]
1.5100 (estimate) | [storage temp. ]
2-8°C
| [form ]
crystalline
| [pka]
8.64(at 25℃) | [color ]
off-white to tan
| [Stability:]
Stable, but may be light-sensitive. Incompatible with acids, bases, oxidizing agents. Store at-20C. | [Water Solubility ]
Soluble in alkali and dilute hydrochloric acid. Slightly soluble in water, ethanol and diethyl ether. | [Sensitive ]
Air & Light Sensitive | [Merck ]
14,6695 | [BRN ]
4231961 | [CAS DataBase Reference]
51-41-2(CAS DataBase Reference) | [EPA Substance Registry System]
51-41-2(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T+,C,F | [Risk Statements ]
R26/27/28:Very Toxic by inhalation, in contact with skin and if swallowed .
R34:Causes burns.
R11:Highly Flammable. | [Safety Statements ]
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S16:Keep away from sources of ignition-No smoking . | [RIDADR ]
UN 2811 6.1/PG 2
| [WGK Germany ]
3
| [RTECS ]
DN5950000
| [F ]
8-10-23 | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
29225090 | [Safety Profile]
Poison by ingestion,
intraperitoneal, subcutaneous, and
intravenous routes. An experimental
teratogen. Experimental reproductive
effects. Human mutation data reported. A
sympathomimetic vasopressor. When heated
to decomposition it emits toxic fumes of
NOx. | [Hazardous Substances Data]
51-41-2(Hazardous Substances Data) | [Toxicity]
dni-hmn:oth 5800 nmol/L CNREA8 40,1414,80 |
| Hazard Information | Back Directory | [Hazard]
May cause local tissue necrosis, headache,
bradycardia, hypertension; poison; teratogen; mutagen. | [Chemical Properties]
solid | [Uses]
An adrenergic neurotransmitter. | [Uses]
Norepinephrine is used for increasing cardiac constriction and for the necessary eleva�tion of blood pressure after sharp decline, which can result from surgical intervention or
trauma. | [Uses]
Vascular active drug resistance to shock | [Definition]
ChEBI: The R-enantiomer of noradrenaline. | [Brand name]
Levophed (Hospira);Noradrec;Xylotox. | [World Health Organization (WHO)]
Vasoconstrictor agents have been in use for many years to
prolong duration of action of local anaesthetics, particularly in dentistry.
Combination products containing epinephrine or levarterenol in concentrations of
1:80,000 or less remain widely available. See also WHO comment for epinephrine. | [Biological Functions]
Most central noradrenergic neurons are located in the
nucleus locus ceruleus of the pons and in neurons of
the reticular formation. Fibers from these nuclei innervate
a large number of cortical, subcortical, and spinomedullary
fields. Many functions have been ascribed to
the central noradrenergic neurons, including a role in affective disorders (see Chapter 33), in learning and
memory, and in sleep–wake cycle regulation.The mammalian
CNS contains both α- and β-adrenoceptors. | [General Description]
Norepinephrine (NE, Levophed) differs from DA onlyby addition of a 1-OH substituent (β-OH-DA) and from Eonly by lacking the N-methyl group. Like DA, it is polar andrapidly metabolized by both COMT and MAO, resulting inpoor oral bioavailability and short DOA (1 or 2 minutes evenwhen given intravenously). It is a stimulant of α1-, α2-, andβ1-adrenoceptors (notice that lacking the N-methyl group resultsin lacking β2- and β3-activity). It is used to counteractvarious hypotensive crises, because its α-activity raisesblood pressure and as an adjunct treatment in cardiac arrestbecause its β-activity stimulates the heart. It has limitedclinical application caused by the nonselective nature of itsactivities. | [Biochem/physiol Actions]
Adrenergic neurotransmitter | [Pharmacology]
Norepinephrine is the primary neurotransmitter produced and released by adrenergic neu�rons, and in literature it is also described as and called (�) noradrenaline or levarterenol.
This vasopressor catecholamine reduces both the resistance and capacity of blood vessels
by stimulating α-adrenoreceptors and having a direct cardiostimulatory effect, which is
accomplished by activation of β1-adrenoreceptors. Norepinephrine exhibits significantly
less activity than epinephrine as a drug for widening blood vessels through the activation
of β2-adrenoreceptors. Elevation of both stylistic and diastolic blood pressure is a typical
reaction to intravenous introduction of norepinephrine. | [Synthesis]
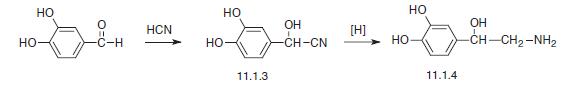
Norepinephrine, L-1-(3,4-dihydroxyphenyl)-2-aminoethanol (11.1.4), is synthesized by two methods starting from 3,4-dihydroxybenzaldehyde. According to the first method, the indicated aldehyde is transformed into the cyanohydrin (11.1.3) by reaction with hydrogen cyanide, which is then reduced into norepinephrine (11.1.5).
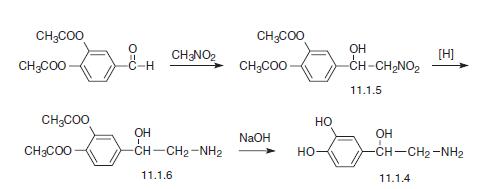
The second method consists of the condensation of diacetate of the same aldehyde with nitromethane, which forms (3,4-diacetoxyphenyl)-2-nitroethanol (11.1.5). Then the nitro group is reduced and the product (11.1.6) is hydrolyzed into the desired norepinephrine (11.1.4) [4,9,13,14].
| [Purification Methods]
Recrystallise adrenor from EtOH and store it in the dark under N2. [pKa, Lewis Brit J Pharmacol Chemother 9 488 1954, UV: Bergstr.m et al. Acta Physiol Scand 20 101 1950, Fluorescence: Bowman et al. Science NY 122 32 1955, Tullar J Am Chem Soc 70 2067 1948.] The L-tartrate salt monohydrate has m 102-104.5o, [] D -11o (c 1.6, H2O), after recrystallisation from H2O or EtOH. [Beilstein 13 III 2382.] |
|
|