| Identification | More | [Name]
Fluoxetine hydrochloride | [CAS]
56296-78-7 | [Synonyms]
FLUOXETINE-D5 HCL
FLUOXETINE-D5 HCL (PHENYL-D5)
FLUOXETINE-D5 HYDROCHLORIDE
(+-)-methyl-gamma-(4-(trifluoromethyl)phenoxy)benzenepropanaminehydrochlorid
3-(p-trifluoromethylphenoxy)-n-methyl-3-phenylpropylaminehydrochloride
fontex
lilly110140
n-methyl-3-phenyl-3-(p-trifluoromethylphenoxy)-propylaminhydrochloride
methyl[3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]ammonium chloride
(±)-N-methyl-γ-[4-(trifluoromethyl)phenoxy]-benzenepropanamine
Adofen-d5
Fluctin-d5
Fluoxeren-d5
Fontex-d5
Foxetin-d5
Lovan-d
LY-110140-d5
N-methyl-g-[4-(trifluoromethyl)phenoxy]benzene-d5-propanamine Hydrochloride
Fluoxetine hydrochloride
Methyl[3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]amine hydrochloride | [EINECS(EC#)]
260-101-2 | [Molecular Formula]
C17H19ClF3NO | [MDL Number]
MFCD03428208 | [Molecular Weight]
345.79 | [MOL File]
56296-78-7.mol |
| Chemical Properties | Back Directory | [Appearance]
White to Off-White Powder | [Melting point ]
158-159°C | [alpha ]
-0.05~+0.05° | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
H2O: soluble (sparingly) | [form ]
solid | [color ]
white | [Water Solubility ]
Soluble in dimethylsulfoxide and water. | [Usage]
A selectiive derotonin reuptake inhibitor. Used as an antidepressant | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 3 months. | [InChIKey]
GIYXAJPCNFJEHY-UHFFFAOYSA-N | [CAS DataBase Reference]
56296-78-7(CAS DataBase Reference) | [EPA Substance Registry System]
56296-78-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R38:Irritating to the skin.
R41:Risk of serious damage to eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
3249 | [WGK Germany ]
3 | [RTECS ]
UI4050000 | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29222990 | [Hazardous Substances Data]
56296-78-7(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Description]
Fluoxetine hydrochloride is a serotonin-selective antidepressant approved for the treatment of major depressive disorders, including those with concomitant anxiety.
It also appears effective in the treatment of obesity. | [Chemical Properties]
White to Off-White Powder | [Originator]
Lilly (USA) | [Uses]
A labelled selective serotonin reuptake inhibitor (SSRI). Used as an antidepressant. | [Uses]
A selectiive derotonin reuptake inhibitor. Used as an antidepressant | [Uses]
A selective serotonin reuptake inhibitor (SSRI). Used as an antidepressant. | [Application]
Fluoxetine hydrochloride has been used to study its effect on the binding ability of the radiopharmaceutical 123I-labeled 2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine ([123I]ADAM) to SERT (serotonin transporters) in mice. It has also been used for the chronic treatment of light deprived animals. | [Definition]
ChEBI: Fluoxetine hydrochloride (1:1) is a hydrochloride and a N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine. It inhibits the reuptake of serotonin and is used chiefly as an antidepressant. | [Preparation]
A method for the synthesis of fluoxetine hydrochloride was developed by the Mannich reaction of acetophenone with methylamine hydrochloride, paraformaldehyde to form 3-methylamino-1-phenylacetic acid hydrochloride, followed by the preparation of 3-methylamino-I-phenylpropanol in methanol with potassium borohydride reductant, followed by etherification and salt formation to synthesize the antidepressant fluoxetine monohydrochloride.
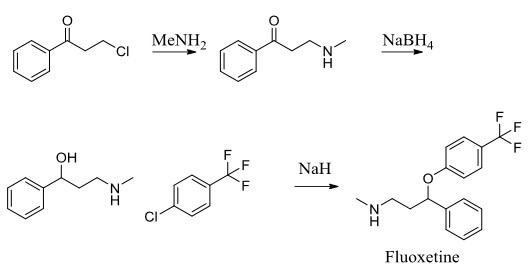
Fluoxetine is the active ingredient in the antidepressant Prozac. It works as a selective serotonin reuptake inhibitor to treat conditions including depression and obsessive-compulsive disorder. To assemble this molecule, a three-step synthesis was utilized. Intermediates included 1- propanone, 3-(methylamino)-1-phenyl-(synthesized through an SN2 reaction between 3-chloropropiophenone and methylamine) and α-[2- (methylamino) ethyl] benzyl alcohol (synthesized through reduction of the first intermediate using NaBH4). The second intermediate was subjected to 4-chlorobenzotrifluoride and sodium hydride to produce the desired molecule, fluoxetine. | [Manufacturing Process]
About 600 g of β-dimethylaminopropiophenone hydrochloride were converted to the corresponding free base by the action of 1.5 N aqueous sodium hydroxide. Free base was dissolved in 2 L of THF, and the resulting solution added in dropwise fashion to a solution of 4 moles of diborane in 4 L of THF. The reaction mixture was stirred overnight. An additional mole of diborane in 1 L of THF was added, and the reaction mixture stirred again overnight. Next, 2 L of aqueous hydrochloric acid were added to decompose any excess diborane present. The tetrahydrofuran was removed by evaporation. The acidic solution was extracted twice with 1 L portions of benzene, and the benzene extracts were discarded. The acidic solution was then made basic with an excess of 5 N aqueous sodium hydroxide. The basic solution was extracted three times with 2 L portions of benzene. The combined extracts washed with a saturated aqueous sodium chloride and then dried. Evaporation of the solvent in vacuo yields 442 g of N,N-dimethyl-3-phenyl-3hydroxypropylamine.
A solution containing 442 g of N,N-dimethyl-3-phenyl-3-hydroxypropylamine in 5 L of chloroform was saturated with dry gaseous hydrogen chloride. 400 mL of thionyl chloride were then added to the solution at a rate sufficient to maintain reflux. The solution was refluxed an additional 5 hours. Evaporation of the chloroform and other volatile constituents in vacuo yielded N,Ndimethyl-3-phenyl-3-chloropropylamine hydrochloride which was collected by filtration, and the filter cake washed twice with 1.5 L portions of acetone. The washed crystals weighed about 500 g and melted at 181-183°C with decomposition. An additional 30 g of compound were obtained from the acetone wash by standard crystallization procedures. The structure of the above compound was verified by NMR and titration.
A solution of 50 g p-trifluoromethylphenol, 12 g of solid sodium hydroxide and 400 mL of methanol were added 29.8 g of N,N-dimethyl 3-phenyl-3chloropropylamine hydrochloride. The resulting reaction mixture was refluxed for about 5 days and then cooled. The methanol was removed by evaporation, and the resulting residue taken up in a mixture of ether and 5 N aqueous sodium hydroxide. The ether layer was separated and washed twice with 5 N aqueous sodium hydroxide and three times with water. The ether layer was dried, and the ether removed by evaporation in vacuo to yield as a residue N,N-dimethyl-3-(p-trifluoromethylphenoxy)-3-phenylpropylamine.
To a solution 8.1 g of cyanogen bromide in 500 mL benzene and 50 mL of toluene at 5°C in nitrogen was added dropwise a solution of 12.146 g of N,Ndimethyl-3-(p-trifluoromethylphenoxy)-3-phenylpropylamine in 40 mL of benzene. The temperature of the reaction mixture was allowed to rise slowly to room temperature, at which temperature stirring was continued overnight while still maintaining a nitrogen atmosphere. 100 mL of benzene were added. The reaction mixture was washed twice with water, once with 2 N aqueous sulfuric acid and then with water until neutral. The organic layer was dried, and the solvents removed therefrom by evaporation in vacuo to yield about 9.5 g of an oil comprising N-methyl-N-cyano-3-(p-trifluoromethylphenoxy)-3phenylpropylamine.
The reaction mixture containing 100 g potassium hydroxide, 85 mL water, 400 mL ethylene glycol and 9.50 g of N-methyl-N-cyano-3-(ptrifluoromethylphenoxy)-3-phenylpropylamine was heated to refluxing temperature for 20 hours, and was then cooled. 500 mL of water were added. The reaction mixture was extracted with three 500 mL portions of ether. The combined extracts washed with water. The water wash was discarded. The ether solution was next contacted with 2 N aqueous hydrochloric acid. The acidic aqueous layer was separated. A second aqueous acidic extract with 2 N hydrochloric acid was made followed by three aqueous extracts and an extract with saturated aqueous sodium chloride. The aqueous layers were all combined and made basic with 5 N aqueous sodium hydroxide. N-methyl 3(p-trifluoromethylphenoxy)-3-phenylpropylamine, formed in the above reaction, was insoluble in the basic solution and separated. The amine was extracted into ether. Two further ether extractions were carried out. The ether extracts were combined, and the combined extracts washed with saturated aqueous sodium chloride and then dried. Evaporation of the ether in vacuo yielded about 6.3 g of N-methyl-3-(p-trifluoromethylphenoxy)-3phenylpropylamine. The amine free base was converted to the corresponding hydrochloride salt. | [Brand name]
Prozac (Lilly); Sarafem (Lilly); Sarafem (Warner Chilcott). | [Therapeutic Function]
Antidepressant | [General Description]
Fluoxetine hydrochloride is an antidepressant drug and a selective serotonin reuptake inhibitor, which is widely used for the treatment of major depressive disorders, obsessive-compulsive disorder and panic fits. | [Biological Activity]
Selective serotonin reuptake inhibitor. Binds to the human 5-HT transporter with a K i of 0.9 nmol/l and is between 150- and 900- fold selective over 5-HT 1A , 5-HT 2A , H 1 , α 1 , α 2 -adrenergic, and muscarinic receptors. Antidepressant. Also available as part of the Serotonin Uptake Inhibitor Tocriset™ . | [Biochem/physiol Actions]
Selective serotonin reuptake inhibitor; antidepressant. | [Side effects]
Common adverse reactions are dry mouth, loss of appetite, nausea, insomnia, fatigue, a few cases of anxiety and headaches are seen . Because fluoxetine hydrochloride has longer half-life, patients whose liver and kidney function are poor or elderly patients, should appropriately reduce the dose. Children should be applied in accordance with the doctor's advice. Patients who has the history of epilepsy, pregnancy or lactation women should use with caution . If rash or fever occurs, the drug should be discontinued immediately and symptomatic treatment should be given. It is not appropriate to be used with monoamine oxidase inhibitor (MAOI) ; if necessary, the drug should be discontinued after five weeks, before switching to a monoamine oxidase inhibitor (MAOI). | [in vitro]
in xenopusoocytes expressing either cloned 5ht2c receptors or 5ht receptors, micromolar concentrations of fluoxetine (prozac) inhibited the membrane currents elicited by serotonin (5-hydroxytryptamine; 5ht). for responses elicited by 1 μm 5-ht, the ic50 of fluoxetine was about 20 μm. fluoxetine also inhibited the binding of [3h]5ht to 5ht2c receptors expressed in hela cells and the binding of [3h]5ht to 5ht receptors in rat cortex membranes, with ki of ≈65–97 nm and ≈ 56 μm, respectively[2]. administration of fluoxetine blocked the downregulation of cell proliferation of hippocampal cells resulting from inescapable shock (is), which resulted in a state of behavioral despair[3]. fluoxetine increased the number of newborn cells in the dentate gyrus of the hippocampus of adult rat. fluoxetine also increased the number of proliferating cells in the prelimbic cortex[4]. fluoxetine accelerated the maturation of immature neurons. fluoxetine enhanced neurogenesis-dependent long-term potentiation (ltp) in the dentate gyrus [5]. fluoxetine, but not other selective serotonin uptake inhibitors such as citalopram, fluvoxamine, paroxetine and sertraline, increased norepinephrine and dopamine extracellular levels in prefrontal cortex. fluoxetine produced robust and sustained increases in extracellular concentrations of norepinephrine and dopamine after acute systemic administration [6]. | [in vivo]
fluoxetine reversed the deficit in escape latency observed in animals exposed to inescapable shock in adult male sprague–dawley rats [3].the combination of olanzapine and fluoxetine produced robust, sustained increases of extracellular levels of dopamine ([da](ex)) and norepinephrine ([ne](ex)) up to 361 ± 28% and 272 ± 16% of the baseline, respectively[7]. fluoxetine (5 and 10 mg/kg) reduced cocaine infusions (0.2 mg/kg), and cocaine infusions returned to baseline levels within 48 hr after fluoxetine treatments were terminated [8]. in sham-operated or adrenalectomized/castrated (adx/cx) male rats, administration of fluoxetine dose-dependently (2.9-58 mumol/kg i.p.) increased the brain content of the neurosteroid 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone, 3 alpha, 5 alpha-th prog)[9]. | [storage]
Room temperature | [References]
1) Tatsumi?et al.?(1997),?Pharmacological profile of antidepressants and related compounds at human monoamine transporters; Eur. J. Pharmacol.,?340?249
2) Benfield?et al.?(1986),?Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness; Drugs,?32?481
3) Benvenuto?et al.?(2013),?Pharmacotherapy of autism spectrum disorders; Brain Dev.,?35?119
4) Liu?et al.?(2018),?Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NFkB signaling pathway; J. Neuroinflammation,?15?347
5) Chang?et al.?(2010),?Increased cellular turnover in response to fluoxetine in neuronal precursors derived from human embryonic stem cells; Prog. Int. J. Dev. Biol.,?54?707 |
|
|