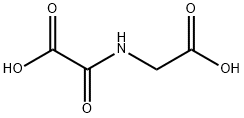
N-OXALYL GLYCINE
- CAS No.
- 5262-39-5
- Chemical Name:
- N-OXALYL GLYCINE
- Synonyms
- NOG;SYM1;SYNS1;N-OXALYL GLYINE;N-OXALYL GLYCINE;Mouse bearing tumor;N-Oxalylglycine (NOG);In vivo drug screening;Tumor modeling services;Human NOG Protein, Fc Tag
- CBNumber:
- CB0250467
- Molecular Formula:
- C4H5NO5
- Molecular Weight:
- 147.09
- MDL Number:
- MFCD00913253
- MOL File:
- 5262-39-5.mol
- MSDS File:
- SDS
| Density | 1.638±0.06 g/cm3(Predicted) |
|---|---|
| storage temp. | Sealed in dry,Room Temperature |
| solubility | deionized water: >10mg/mL |
| form | solid |
| pka | 2.83±0.20(Predicted) |
| FDA UNII | VVW38EB8YS |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symbol(GHS) |  GHS07 |
|||||||||
| Signal word | Warning | |||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 22 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | IRRITANT | |||||||||
| NFPA 704 |
|
N-OXALYL GLYCINE price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | O9390 | N-Oxalylglycine ≥98% (HPLC) | 10MG | $106 | 2024-03-01 | Buy | |
| Sigma-Aldrich | O9390 | N-Oxalylglycine ≥98% (HPLC) | 50MG | $419 | 2024-03-01 | Buy | |
| Cayman Chemical | 13944 | N-Oxalylglycine ≥98% | 5262-39-5 | 5mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 13944 | N-Oxalylglycine ≥98% | 5262-39-5 | 10mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 13944 | N-Oxalylglycine ≥98% | 5262-39-5 | 50mg | $116 | 2024-03-01 | Buy |
N-OXALYL GLYCINE Chemical Properties,Uses,Production
Uses
Oxalylglycine is a broad-spectrum 2OG oxygenase inhibitor that is used in studies of the hypoxic response and chromatin modifications in animals. Oxalylglycine is present as a natural product in rhubarb and spinach leaves. It is also used as a reagent in the synthesis of bipyridinedicarboxylates which are inhibitors of CP4H and therefore may be used in the treatment of fibrotic diseases and metastatic breast cancer.
Uses
The jumonji domain-containing protein 2 (JMJD2) subfamily of histone demethylases have been shown to catalyze demethylation of the methylated forms of histone 3 lysine 9 (H3K9) and H3K36 in vitro and in cells. Because histone demethylases are implicated in certain diseases, including cancer, selective inhibitors are candidate anticancer agents as well as potential tools for elucidating the biological functions of JMJDs. N-Oxalylglycine, the amide analog of α-ketoglutarate, is a cell permeable inhibitor of α-ketoglutarate-dependent enzymes, including JMJD2A, JMJD2C, and JMJD2E (IC50s = 250, 500, and 24 μM, respectively). It can also inhibit the prolyl hydroxylase domain-containing proteins PHD1 and PHD2 with IC50 values of 2.1 and 5.6 μM, respectively.[Cayman Chemical]
Definition
ChEBI: An amino dicarboxylic acid that is iminodiacetic acid with an oxo substituent. It is used as an inhibitor of alpha-ketoglutarate dependent (EC 1.14.11.*) enzymes.
Biological Activity
n-oxalylglycine, as known as nog, is a cell permeable inhibitor of α-ketoglutarate-dependent enzymes which include jmjd2a, jmjd2c, and jmjd2e. nog also blocks the prolyl hydroxylase domain-containing proteins phd1 and phd2. demethylation of the methylated forms of histone 3 lysine 9 (h3k9) and h3k36 can be catalyzed by jmjd2 subfamily of histone demethylases. since histone demethylases are related to certain diseases, including cancer, selective inhibitors functions as anticancer agents and potential tools for clarifying the biological functions of jmjds.
Biochem/physiol Actions
N-Oxalylglycine is an inhibitor of α-ketoglutarate-dependent enzymes and mimics the initial steps but does not initiate the hydroxylation process. N-Oxalylglycine has been used to inhibit Jumonji C-domain-containing histone lysine demethylases.
in vitro
nog was identified as a natural product and presented in plants, including rheum rhabarbarum (rhubarb) and spinach oleracea (spinach) leaves. however, there was no nog observed human embryonic kidney cells (hek 293t) or escherchia coli. nog regulated gene expression via blocking 2-oxoglutarate-dependent oxygenases [1].
IC 50
250 μm: inhibits α-ketoglutarate-dependent enzyme jumonji domain-containing protein 2 (jmjd2) a.
References
[1]. al-qahtani, k., jabeen, b., sekirnik, r., riaz, n., claridge, t., schofield, c., & mccullagh, j. the broad spectrum 2-oxoglutarate oxygenase inhibitor n-oxalylglycine is present in rhubarb and spinach leaves. phytochemistry. 2015; 117: 456-461.
N-OXALYL GLYCINE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 28302 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9635 | 58 |
| CR Corporation Lomited | fred.wen@crcorporation.cn | CHINA | 12069 | 58 | |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Sichuan Biosynce Pharmatech Co., Ltd. | +8619950309693 | diane@biosynce.com | China | 4874 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33350 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |