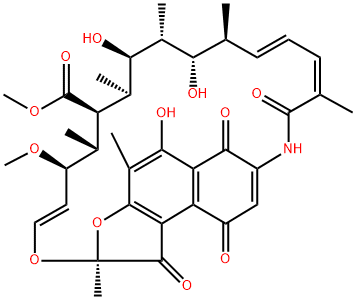
Rifamycin S
- CAS No.
- 13553-79-2
- Chemical Name:
- Rifamycin S
- Synonyms
- rifamycin;100722;rifomycins;nci144-130;RIFAMYCIN S;RIFAMPICIN S;Rifamycin S CRS;Rifamycin S USP/EP/BP;Rifaximin EP Impurity E;Rifamycin EP Impurity B
- CBNumber:
- CB1187968
- Molecular Formula:
- C37H45NO12
- Molecular Weight:
- 695.75
- MDL Number:
- MFCD06198807
- MOL File:
- 13553-79-2.mol
- MSDS File:
- SDS
| Melting point | 179-181°C (dec.) |
|---|---|
| alpha | D20 +476° (c = 0.1 in methanol) |
| Boiling point | 700.89°C (rough estimate) |
| Density | 1.2387 (rough estimate) |
| refractive index | 1.6630 (estimate) |
| storage temp. | -20°C Freezer |
| solubility | Benzene (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| pka | 3.85±0.70(Predicted) |
| color | Orange to Dark Orange |
| λmax | 390nm(MeOH)(lit.) |
| Merck | 14,8217 |
| InChIKey | BTVYFIMKUHNOBZ-ODRIEIDWSA-N |
| FDA UNII | PI53N820JV |
| ATC code | A07AA13,D06AX15,J04AB03,S01AA16,S02AA12 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H371 | |||||||||
| Precautionary statements | P501-P260-P270-P264-P308+P311-P405 | |||||||||
| RTECS | KD1925000 | |||||||||
| HS Code | 2941.90.1050 | |||||||||
| Toxicity | LD50 in mice (mg/kg): 122 i.v.; 258 i.p.; 3000 orally (Sensi, 1964) | |||||||||
| NFPA 704 |
|
Rifamycin S price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | R0950000 | Rifamycin S European Pharmacopoeia (EP) Reference Standard | 13553-79-2 | r0950000 | $220 | 2024-03-01 | Buy |
| TCI Chemical | R0200 | Rifamycin S >98.0%(HPLC) | 13553-79-2 | 1g | $73 | 2024-03-01 | Buy |
| TCI Chemical | R0200 | Rifamycin S >98.0%(HPLC) | 13553-79-2 | 5g | $220 | 2024-03-01 | Buy |
| TRC | R508210 | Rifamycin S | 13553-79-2 | 50mg | $160 | 2021-12-16 | Buy |
| ChemScene | CS-0090973 | Rifamycin S 99.22% | 13553-79-2 | 100mg | $150 | 2021-12-16 | Buy |
Rifamycin S Chemical Properties,Uses,Production
Uses
Rifamycin S (Rifaximin EP Impurity E) is a semi-synthetic antibiotic.
Definition
ChEBI: Rifamycin S is an organic molecular entity.
Pharmaceutical Applications
The rifamycins are a family of antibiotics produced by an actinomycete now classified as Amycolatopsis mediterranei. All the therapeutically useful rifamycins are semisynthetic derivatives of rifamycin B, a fermentation product that is poorly active, but easily produced and readily converted chemically into rifamycin S, from which most active derivatives are prepared. They all share the general structure.
Natural products like rifamycins, which are characterized by an aromatic ring spanned by an aliphatic bridge (ansa) are called ‘ansamycins’. To this class belong the streptovaricins and the tolypomycins (chemically and biologically similar to rifamycins) and geldanamycin and the maytansines, which have quite different, antiblastic, biological activities. Among the vast number of rifamycin derivatives investigated, rifampicin (rifampin) is by far the most important and most widely used. Various others, notably rifabutin, rifapentine and rifaximin, are also in use in various parts of the world. Rifamycin SV and rifamide are much less widely available.
Interest in these antibiotics centers on their potent activity against pathogenic Gram-positive cocci and mycobacteria. Knowledge of the general properties of the group is largely based on extensive study and use of rifampicin but, insofar as they have been investigated, the main features are exhibited also by the other congeners:? Bactericidal action through inactivation of bacterial DNAdependent RNA polymerase ? Mechanism of resistance consisting of mutation of specific amino acids in the β-chain of RNA polymerase
? Relatively high frequency of resistant mutants; resistance is not horizontally transferable ? Significant biliary excretion and stimulation of hepatic metabolism. The structure of RNA polymerase is highly conserved among bacteria and when tested in cell-free systems all rifamycins present similar intrinsic activity. Differences in the minimum inhibitory concentration (MIC) values among the various congeners are caused by different abilities to penetrate into cells. Rifamycins also inhibit the RNA polymerase of eukaryotic organelles, such as mitochondria, since these are of a prokaryotic type. Some rifamycins carrying a large lipophilic chain inhibit eukaryotic RNA and DNA polymerases and viral reverse transcriptases. These effects have no clinical significance.
The different congeners differ substantially in their pharmacokinetic behavior and in their therapeutic efficacy. The principal use of rifampicin and rifapentine is in the treatment of tuberculosis and leprosy. Rifabutin is approved for the prevention of mycobacterial infections in AIDS patients. Rifampicin proved so important in the treatment of tuberculosis that in many countries its use was restricted to that indication for fear that more widespread use would encourage the emergence of resistant Mycobacterium tuberculosis strains. Those fears have proven to be exaggerated and interest has been increasingly refocused on what was originally anticipated to be an important use: treatment of severe Gram-positive infections. To prevent emergence of resistance, co-administration of another effective agent is required.
Rifaximin does not encourage emergence of resistance in mycobacteria and is used in the treatment of gastrointestinal infections. Rifamycin SV and rifamide were originally released for the treatment of infections with susceptible Gram-positive organisms and infections of the biliary tract.
Clinical Use
The rifamycins are a group of chemically related antibioticsobtained by fermentation from cultures of Streptomycesmediterranei. They belong to a class of antibiotics called theansamycins that contain a macrocyclic ring bridged acrosstwo nonadjacent positions of an aromatic nucleus. The termansa means “handle,” describing well the topography of thestructure. The rifamycins and many of their semisynthetic derivativeshave a broad spectrum of antimicrobial activity.They are most notably active against Gram-positive bacteriaand M. tuberculosis. However, they are also active againstsome Gram-negative bacteria and many viruses. Rifampin, asemisynthetic derivative of rifamycin B, was released as anantitubercular agent in the United States in 1971. A secondsemisynthetic derivative, rifabutin, was approved in 1992 forthe treatment of atypical mycobacterial infections.
The chemistry of rifamycins and other ansamycins hasbeen reviewed. All of the rifamycins (A, B, C, D, and E) arebiologically active. Some of the semisynthetic derivatives ofrifamycin B are the most potent known inhibitors of DNAdirectedRNA polymerase in bacteria, and their action isbactericidal. They have no activity against the mammalianenzyme. The mechanism of action of rifamycins as inhibitorsof viral replication appears to differ from that for their bactericidalaction. Their net effect is to inhibit the formation of thevirus particle, apparently by preventing a specific polypeptideconversion.77 Rifamycins bind to the β subunit of bacterialDNA-dependent RNA polymerases to prevent chain initiation.78 Bacterial resistance to rifampin has been associatedwith mutations leading to amino acid substitution in the subunit.78 A high level of cross-resistance between variousrifamycins has been observed.
Rifamycin S Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12468 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15956 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21688 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8829 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
View Lastest Price from Rifamycin S manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-07 | Rifamycin S
13553-79-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-12-24 | Rifamycin S
13553-79-2
|
US $16.00 / kg | 1kg | 99% | 1000kg | Hebei Duling International Trade Co. LTD | |
 |
2021-11-02 | Rifamycin S
13553-79-2
|
US $254.00 / KG | 1KG | 98% | 500kg | Baoji Guokang Healthchem co.,ltd |
-

- Rifamycin S
13553-79-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Rifamycin S
13553-79-2
- US $16.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Rifamycin S
13553-79-2
- US $254.00 / KG
- 98%
- Baoji Guokang Healthchem co.,ltd