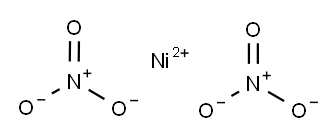
NICKEL NITRATE
- CAS No.
- 13138-45-9
- Chemical Name:
- NICKEL NITRATE
- Synonyms
- NI(NO3)2;nickelous;NICKEL NITRATE;Nickle Nitrate;nickeldinitrate;Nickle dinitrate;Nickel(II)-nitrat;nickel(2+)nitrate;nickelbis(nitrate);NICKEL (II) NITRATE
- CBNumber:
- CB2122997
- Molecular Formula:
- N2NiO6
- Molecular Weight:
- 182.7
- MDL Number:
- MFCD00011139
- MOL File:
- 13138-45-9.mol
| Melting point | 105-110 °C (decomp) |
|---|---|
| vapor pressure | 0.003Pa at 25℃ |
| solubility | soluble in ethanol |
| form | Liquid |
| color | Green |
| Water Solubility | g/100g solution H2O: 44.2 (0°C), 50.0 (25°C), 69.2 (99.5°C); solid phase, Ni(NO3)2 ·6H2O (0°C, 25°C), Ni(NO3)2 ·2H2O (99.5°C) [KRU93] |
| Merck | 13,6537 |
| CAS DataBase Reference | 13138-45-9(CAS DataBase Reference) |
| FDA UNII | 50L0S38I2D |
| EPA Substance Registry System | Nickel(II) nitrate (13138-45-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS03,GHS07,GHS08,GHS05,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H332-H341-H360D-H317-H272-H334-H350i-H315-H318-H302-H410-H372 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P280-P305+P351+P338-P310-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P210-P220-P221P280-P370+P378-P501-P261-P285-P304+P341-P342+P311-P501-P264-P280-P302+P352-P321-P332+P313-P362-P260-P264-P270-P314-P501-P261-P271-P304+P340-P312-P264-P270-P301+P312-P330-P501-P273-P391-P501 |
| Hazard Codes | T |
| Risk Statements | 45-52/53-48/20-43-49 |
| Safety Statements | 53-45-61-36/37 |
| RIDADR | UN3264 |
| WGK Germany | 3 |
| HazardClass | 8 |
| PackingGroup | III |
NICKEL NITRATE Chemical Properties,Uses,Production
Uses
Nickel nitrate is used in the preparation of nickel-impregnated catalysts. It also is used to make nickel plates in nickel-cadmium batteries. Other applications are in ceramics to produce brown colors and in preparing nickel oxide.
Preparation
Nickel nitrate hexahydrate may be prepared by several methods based on the reaction of dilute nitric acid on nickel powder, nickel oxide or nickel carbonate. The reaction is exothermic and requires controlled cooling during production. The hexahydrate can be dehydrated to anhydrous salt by treatment with fuming nitric acid.
Chemical Properties
Green, deliquescent crystals.Soluble in water, ammonium hydroxide, and alcohol.
Chemical Properties
Nickel nitrate is a green powder.
Physical properties
The hexahydrate forms emerald green monoclinic crystals; hygroscopic; density 2.05 g/cm3; isomorphous with corresponding cobalt salt; melts at 56.7°C; loses water on heating, decomposing to nickel oxide; very soluble in water; aqueous solution acidic; soluble in ethanol.
Uses
Nickel plating, preparation of nickel catalysts, manufacture of brown ceramic colors.
General Description
NICKEL NITRATE is a green crystalline solid. NICKEL NITRATE is soluble in water. NICKEL NITRATE is noncombustible, but NICKEL NITRATE will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving NICKEL NITRATE. NICKEL NITRATE is used in nickel plating and to make nickel catalysts for use in chemical manufacture.
Air & Water Reactions
Nickel nitrate, Ni(N03)2·SH20, is green, monoclinic, deliquescent crystals that are soluble in water and alcohol; a dangerous fife hazard and strong oxidant that is used in nickel plating and the preparation of catalysts and brown ceramic coloring.
Reactivity Profile
Green, crystalline material, toxic and carcinogenic. A powerful oxidizer, NICKEL NITRATE may cause a violent reaction with reducing materials (e.g., magnesium or aluminum powder, tin(II) chloride). Dangerous fire hazard, acts as oxygen carrier. When heated to decomposition NICKEL NITRATE emits highly toxic fumes of oxides of nitrogen [Lewis, 3rd ed., 1993, p. 915]. Complexes with tetrammine and tetrahydrazine are explosive [Bretherick, 5th ed., 1995, p. 1685].
Hazard
Dangerous fire risk, strong oxidizing agent.
Health Hazard
Inhalation of dust causes irritation of nose and throat. Ingestion causes vomiting. Dust irritates eyes and may cause dermatitis in contact with skin.
Flammability and Explosibility
Non flammable
Safety Profile
Confirmed human carcinogen. Poison by intravenous route. Experimental reproductive effects. Mutation data reported. A powerful oxilzer. When heated to decomposition it emits very toxic fumes of NOx. See also NICKEL COMPOUNDS and NITRATES.
Potential Exposure
Nickel nitrate is used in electroplating, in nickel catalyst production for making other chemicals and the manufacture of brown ceramic colors.
Shipping
UN2725 Nickel Nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
A strong oxidizer. Incompatible with strong acids; sulfur, combustibles, organics, and other easily oxidizable materials. Noncombustible, but it will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
NICKEL NITRATE Preparation Products And Raw materials
13138-45-9(NICKEL NITRATE)Related Search:
1of4