Ingenol
- CAS No.
- 30220-46-3
- Chemical Name:
- Ingenol
- Synonyms
- Ingeno;INGENOL;Inglenook;Ingenol, BR;(-)-Ingenol;Ingenol base;Ingenol >99%;Ingenol USP/EP/BP;INGENOL,HIGHPURITY;Ingenol 30220-46-3
- CBNumber:
- CB4370581
- Molecular Formula:
- C20H28O5
- Molecular Weight:
- 348.43
- MDL Number:
- MFCD27980538
- MOL File:
- 30220-46-3.mol
| Melting point | 153 °C |
|---|---|
| Boiling point | 523.8±50.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO: soluble |
| form | film |
| pka | 12.06±0.70(Predicted) |
| color | colorless |
| FDA UNII | IC77UZI9G8 |
Ingenol price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | I1112 | Ingenol | 30220-46-3 | 10MG | $158 | 2024-03-01 | Buy |
| TCI Chemical | I1112 | Ingenol | 30220-46-3 | 50MG | $475 | 2024-03-01 | Buy |
| Cayman Chemical | 14031 | Ingenol ≥98% | 30220-46-3 | 500μg | $34 | 2024-03-01 | Buy |
| Cayman Chemical | 14031 | Ingenol ≥98% | 30220-46-3 | 1mg | $59 | 2024-03-01 | Buy |
| Cayman Chemical | 14031 | Ingenol ≥98% | 30220-46-3 | 5mg | $249 | 2024-03-01 | Buy |
Ingenol Chemical Properties,Uses,Production
Description
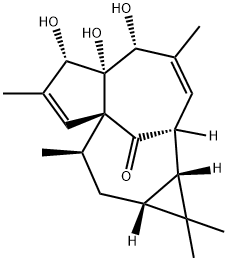
Ingenol is a diterpenoid related to phorbol, derived from the milkweed plant E. peplus.1 It is a protein kinase C activator that displays a Ki value of 30 μM and an ED50 value of 27 μM in vitro. Most ingenol esters are tumor-promoting. However, ingenol mebutate possesses anti-tumor activity when used topically for actinic keratosis.
Chemical Properties
Isolated in 1968, ingenol, a highly oxygenated tetracyclic diterpene classified as a member of the phorboid family, is the parent compound of several dozen naturally occurring ingenanes that possess the same carbon skeleton but varied peripheral functionalities. In addition to their intriguing “inside-outside” bridged BC ring system, the ingenanes display interesting biological profiles that range from tumorpromoting to anti-leukemic and anti-HIV activities. Since the early 1980s, this combination of important biological function and complex architecture has inspired the efforts of numerous synthetic chemists.4 Previously, we reported an approach toward 1 wherein known â-ketoester 2 was advanced to diene 3, which served as a ring-closing metathesis substrate in a first generation Grubbs reaction that delivers the ingenane tetracycle 4.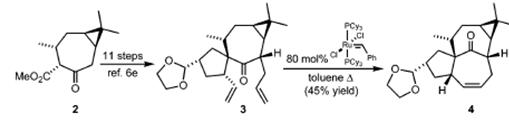
Ingenol mebutate
Ingenol mebutate is a selective small-molecule activator of protein kinase C (PKC) isolated from the plant Euphorbia peplus with potential antineoplastic activity. Ingenol mebutate activates various protein kinase C (PKC) isoforms, thereby inducing apoptosis in some tumor cells, including myeloid leukemia cells, melanoma cells, and basal cell carcinoma cells. The PKC family consists of signaling isoenzymes that regulate many cell processes including proliferation, differentiation, and apoptosis.
Ingenol mebutate was approved by the FDA in January 2012, and it is marketed under the name Picato®. Picato gel is indicated for the topical treatment of actinic keratosis. Before approval, ingenol mebutate was called PEP005 as an investigational drug. PEP005 is a selective small molecule activator of protein kinase C (PKC) extracted from the plant Euphorbia peplus, whose sap has been used as a traditional medicine for the treatment of skin conditions including warts and cancer. PEP005 also has potent anti-leukemic effects, inducing apoptosis in myeloid leukemia cell lines and primary AML cells at nanomolar concentrations.
Ingenol mebutate gel (Picato) is a topical cream that the manufacturer is requesting to use as a secondline treatment in patients with actinic keratosis (AK) who have failed or are intolerant to 5-fluorouracil (5-FU). Ingenol mebutate gel is available in two strengths – a 0.015% dose for lesions on the face and scalp and a 0.05% dose for lesions on the trunk and extremities.
Pharmacokinetics & metabolism
The pharmacokinetics investigation of the systemic absorption of ingenol mebutate gel 0.05% was evaluated in a randomized vehicle-controlled double blind study, in which 1 g was applied to a contiguous 100 cm2 area of multiple AKs on the dorsal forearms of 16 patients in two consecutive daily applications. This clinical trial showed that there was no detectable systemic absorption of the parent drug or its two principal metabolites (both acyl isomers of ingenol mebutate), when the limit of detection was 0.1 ng/ml.
In vitro studies using [3H]-ingenol mebutate showed that metabolism of the drug by human hepatocytes is extensive. Other in vitro studies showed that ingenol mebutate neither induces human CYPP450 enzymes CYP1A2, 2C9 and 3A4a, nor inhibits CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4.
Description
Ingenol is a diterpenoid related to phorbol, derived from the milkweed plant E. peplus. It is a protein kinase C activator that displays a Ki value of 30 μM and an ED50 value of 27 μM in vitro. Most ingenol esters are tumor-
Uses
Ingenol, is the analogue of Ingenol 3-Angelate (I655800), which has anti-tumor activity when used topically for the treatment of actinic keratosis.
Definition
ChEBI: A tetracyclic diterpenoid that is 1a,2,5,5a,6,9,10,10a-octahydro-1H-2,8a-methanocyclopenta[a]cyclopropa[e][10]annulen-11-one substituted at positions 5, 5a and 6 by hydroxy groups, positions 1, 1, 7 and 9 by met yl groups, position 4 by a hydroxymethyl group and position 1 by an oxo group (the 1aR,2S,5R,5aR,6S,8aS,9R,10aR diastere mer).
Ingenol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3424 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 242 | 58 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9341 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | sales@pioneerbiotech.com | China | 3000 | 58 |
| NanJing Spring & Autumn Biological Engineering CO., LTD. | +8613815430202 | sale02@cqherb.com | CHINA | 376 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8348 | 58 |
Related articles
- Ingenol: pharmacokinetic, mechanism of action and clinical applications
- Ingenol, derived from Euphorbia peplus, shows promise in dermatology for its minimal systemic absorption and diverse therapeut....
- Dec 1,2023
- Total Synthesis of Ingenol
- Isolated in 1968, ingenol (1), a highly oxygenated tetracyclic diterpene classified as a member of the phorboid family, is the....
- Oct 30,2019
View Lastest Price from Ingenol manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-11 | Ingenol
30220-46-3
|
US $0.00-0.00 / kg | 0.10000000149011612kg | ≥98% | 20tons | Chongqing Zhihe Biopharmaceutical Co., Ltd. | |
 |
2023-06-15 | Ingenol
30220-46-3
|
US $1788.00 / KG | 1KG | 99% | 100kg | Baoji Guokang Bio-Technology Co., Ltd. | |
 |
2023-04-04 | Ingenol
30220-46-3
|
US $400.00 / KG | 1KG | 99% | 10kg | Nanjing Sky Hope Tongyuan Biological Engineering Co., Ltd. |