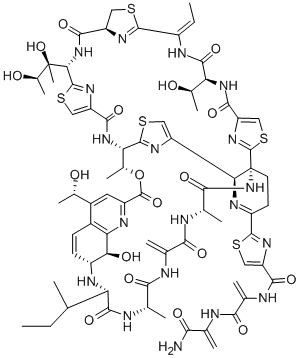
THIOSTREPTON
- CAS No.
- 1393-48-2
- Chemical Name:
- THIOSTREPTON
- Synonyms
- x146;a-8506;thiactin;bryamycin;hiostrepton;THIOSTREPTON;Thiostreptin;THIOSTREPTION;antibioticx146;(-)-Nuciferine
- CBNumber:
- CB5204768
- Molecular Formula:
- C72H85N19O18S5
- Molecular Weight:
- 1664.89
- MDL Number:
- MFCD00135828
- MOL File:
- 1393-48-2.mol
| Melting point | 248-257°C (dec.) |
|---|---|
| alpha | D23 -98.5° (glacial acetic acid); -61° (dioxane); -20° (pyridine) |
| Density | 1.0824 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO or chloroform |
| form | Off-white powder |
| pka | 10.43±0.46(Predicted) |
| color | Off-white |
| Water Solubility | 0.24g/L(28 ºC) |
| Merck | 13,9440 |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 week. |
| EWG's Food Scores | 1 |
| FDA UNII | HR4S203Y18 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332 | |||||||||
| Precautionary statements | P271-P261-P280 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | XN6300100 | |||||||||
| F | 10-21 | |||||||||
| HS Code | 29419090 | |||||||||
| Toxicity | LD50 oral in mouse: > 1gm/kg | |||||||||
| NFPA 704 |
|
THIOSTREPTON price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 598226 | Thiostrepton - CAS 1393-48-2 - Calbiochem Thiostrepton, CAS 1393-48-2, is an antibiotic that inhibits protein synthesis by preventing binding of GTP to 50S ribosomal subunit. | 1393-48-2 | 1g | $130 | 2024-03-01 | Buy |
| Sigma-Aldrich | 598226 | Thiostrepton - CAS 1393-48-2 - Calbiochem Thiostrepton, CAS 1393-48-2, is an antibiotic that inhibits protein synthesis by preventing binding of GTP to 50S ribosomal subunit. | 1393-48-2 | 10g | $1010 | 2024-03-01 | Buy |
| Alfa Aesar | J62332 | Thiostrepton, Streptomyces laurentii, 90+% | 1393-48-2 | 1g | $201 | 2024-03-01 | Buy |
| Alfa Aesar | J62332 | Thiostrepton, Streptomyces laurentii, 90+% | 1393-48-2 | 5g | $705 | 2021-12-16 | Buy |
| Cayman Chemical | 19200 | Thiostrepton ≥95% | 1393-48-2 | 1g | $96 | 2024-03-01 | Buy |
THIOSTREPTON Chemical Properties,Uses,Production
Description
The mammalian transcription factor forkhead box M1 (FoxM1) is induced during G1 phase, with expression continuing through S phase and mitosis. Thiostrepton is a natural peptide thiazole antibiotic that inhibits FoxM1 in mammalian cells, preventing the expression of FoxM1-regulated genes, which includes FoxM1 itself. Through this mechanism, thiostrepton prevents proliferation and induces apoptosis in human cancer cells. These effects correlate with the ability of thiostrepton to act as a proteasome inhibitor.
Chemical Properties
Off-White Solid
Uses
Thiostrepton is a macrocyclic antibiotic incorporating thiazoles and other atypical amino acids. Patented in 1961, thiostrepton has been used as an antibiotic and acts by binding to ribosomes to prevent the binding of the EF-G elongation factor and GTP to the 50S ribosomal subunit. Thiostrepton is an inducer of tipA, a gene that controls the bacterial transcription regulators, TipAL and TipAS, that are central regulators in multidrug resistance. Thiostrepton is closely related to siomycin, a recently discovered inhibitor of oncogenic transcription factor, FoxM1.
Uses
A protein synthesis-inhibiting antibiotic
Uses
beta-adrenergic agonist
Uses
Natural antibiotic derived from Streptomyces.
Definition
ChEBI: A heterodetic cyclic peptide, in which the cyclisation step involves a formal lactonisation between the carboxy group of a quinaldic acid-based residue and a secondary alcohol. An antibiotic that inhibits bacterial protein synthesis. Also acts as an antitu or agent.
General Description
A thiazole-containing peptide antibiotic that inhibits protein synthesis by preventing binding of GTP to 50S ribosomal subunit. Inhibits the function of elongation factor G (EF-G) and the dissociation of EF-G from the ribosome. The thiostrepton-resistant gene is also commonly used as a selective marker for recombinant DNA/plasmid technologies.
Biochem/physiol Actions
Primary TargetEF-G
in vitro
thiostrepton inhibits the transcriptional activity and foxm1 expression, and induces strong apoptosis in human cancer cells of different origin that correlates with suppression of foxm1, including leukemia, neuroblastoma, liver cancer, melanoma and prostate cancer cells. thiostrepton binds foxm1 on the promoter site to inhibit transcriptional activity of foxm1 through the foxm1 autoregulation mechanism [1].
in vivo
thiostrepton suppressed tumor growth in a human breast cancer xenograft model. treatment with developed micelle-thiostrepton nanoparticles decreased xenograft tumor growth induced by the human mda-mb-231 breast and hepg2 liver cancer cell lines. these apoptosis activities in drug-treated tumors were correlated with in vivo suppression of oncogenic foxm1 [1].
References
1) Bowen et al. (2005), Interaction of thiostrepton and elongation factor-G with the ribosomal protein L11-binding domain; J. Biol. Chem., 280 2934 2) Gonzalez et al. (2007), Thiostrepton inhibition of tRNA delivery to the ribosome; RNA, 13 2091 3) Kwok et al. (2008), Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression; Mol. Cancer Ther., 7 2022
THIOSTREPTON Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12471 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8822 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3465 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9126 | 58 |
View Lastest Price from THIOSTREPTON manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-30 | THIOSTREPTON
1393-48-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-07-13 | Thiostrepton
1393-48-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-07-10 | Thiostrepton
1393-48-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |
-

- THIOSTREPTON
1393-48-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Thiostrepton
1393-48-2
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Thiostrepton
1393-48-2
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
1393-48-2(THIOSTREPTON)Related Search:
1of4