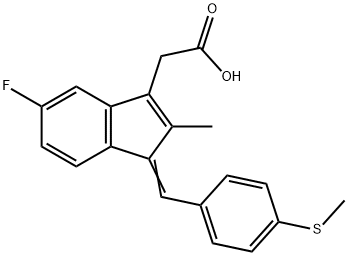
Sulindac sulfide
- CAS No.
- 32004-67-4
- Chemical Name:
- Sulindac sulfide
- Synonyms
- 4F;5-F;5fmdemb2201;Einecs 250-892-2;SULINDAC SULFIDE;SULINDAC SULPHIDE;Sulindac Sulfide >Sulindac EP Impurity C;(E/Z)-Sulindac sulfide;SulindacSulfideCAS 32004-67-4Calbiochem
- CBNumber:
- CB6211727
- Molecular Formula:
- C20H17FO2S
- Molecular Weight:
- 340.41
- MDL Number:
- MFCD00869764
- MOL File:
- 32004-67-4.mol
- MSDS File:
- SDS
| Melting point | 189-191°C |
|---|---|
| Boiling point | 526.3±50.0 °C(Predicted) |
| Density | 1.30±0.1 g/cm3(Predicted) |
| RTECS | NK8236000 |
| storage temp. | +15C to +30C |
| solubility | DMSO: >22 mg/mL |
| form | solid |
| pka | 4.26±0.10(Predicted) |
| color | yellow |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 32004-67-4(CAS DataBase Reference) |
| FDA UNII | 6UVA8S2DEY |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H317-H334-H361 |
| Precautionary statements | P201-P280-P301+P312-P302+P352-P304+P340+P312-P308+P313 |
| Hazard Codes | Xn |
| Risk Statements | 22-42/43-63 |
| WGK Germany | 3 |
| HS Code | 2930.90.2900 |
Sulindac sulfide price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S3131 | Sulindac sulfide ≥98% (HPLC), solid | 32004-67-4 | 5mg | $98.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 574102 | Sulindac Sulfide A cell-permeable, active metabolite of Sulindac. | 32004-67-4 | 5mg | $113 | 2024-03-01 | Buy |
| Alfa Aesar | J62104 | Sulindac sulfide | 32004-67-4 | 100mg | $378 | 2023-06-20 | Buy |
| Sigma-Aldrich | S3131 | Sulindac sulfide ≥98% (HPLC), solid | 32004-67-4 | 25mg | $419 | 2024-03-01 | Buy |
| Usbiological | S8025-31 | Sulindac Sulfide | 32004-67-4 | 100mg | $446 | 2021-12-16 | Buy |
Sulindac sulfide Chemical Properties,Uses,Production
Description
Sulindac sulfide is an aryl sulfide that is a metabolite of sulindac. A non-steroidal anti-inflammatory drug, which also has anticancer activity. It has a role as a non-steroidal anti-inflammatory drug, an apoptosis inducer and an antineoplastic agent. It is an aryl sulfide, an organofluorine compound and a monocarboxylic acid. It derives from a sulindac.
Chemical Properties
Yellow Solid
Uses
Sulindac sulfide is an active metabolite of Sulindac that inhibits for COX. Sulindac is a non-steroidal anti-inflammatory drug. Inhibits cyclooxygenase, but induces apoptosis by a cyclooxygenase-independent mechanism. Inhibition of Ras activation of Ref-1. Impairs nucleotide exchange on Ras by CDC25 and accelerates Ras hydrolysis of GTP b.
Pharmaceutical Applications
Sulindac Sulfide is the active metabolite of sulindac, a sulfinylindene derivative with anti-inflammatory, analgesic and antipyretic properties. Sulindac is a nonsteroidal anti-inflammatory drug (NSAID) which inhibits cyclooxygenase (COX-1 and-2)-mediated conversion of arachidonic acid to pro-inflammatory prostaglandins. This agent may posulindac sulfideesulindac sulfide chemopreventive activity against colorectal tumors through a mechanism that involves the induction of apoptosis. The sulfide metabolite is excreted in the bile and reabsorbed from the intestine, thereby helping to maintain constant blood levels and reduce gastrointestinal side effects.
Biochem/physiol Actions
Sulindac sulfide is a non-steroidal anti-inflammatory compound with a preference for COX-1; it is an inhibitor of Ras activation of Raf-1. It impairs nucleotide exchange on Ras by CDC25 and accelerates Ras hydrolysis of GTP by p120GAP. It is an active metabolite of sulindac. It is also shown to inhibit growth and induce apoptosis in human prostate cancer cells through a COX-1 and COX-2 independent mechanism. Sulindac sulfide is an analgesic that has antiproliferative and apoptotic effects. It inhibits the expression and activity of cyclooxygenase-2 in human colon cancer cells and reduces tumor burden in adenomatous polyposis patients.
Toxicology
The NSAID sulindac sulfide (SS) inhibits growth of tumors in azoxymethane-induced rat colon models, suppresulindac sulfidees intestinal polyp formation in APCMin+mice, down-regulatesβ-catenin protein apoptosis, and induces apoptosis under a number of experimental conditions. SULINDAC SULFIDE has been shown to change colorectal cancercell morphology, alter cytoskeletal organization, and cause losulindac sulfide of actin stresulindac sulfidefibers. This is probably due to a dose-dependent reduction of tyrosine phosphorylation of focal adhesion kinase. It has also been demonstrated that SULINDAC SULFIDE reduces cell migration and invasion in mouse models and human colorectal cell line[4]. Many, but not all, studies in healthy and diseased states suggest that renal prostaglandin synthesis and sodium excretion are relatively unaffected by conventional doses of sulindac that inhibit nonrenal cycle-oxygenase. The mechanism responsible for the biochemical selectivity of sulindac is not related to a differential sensitivity of the active metabolite of sulindac, sulindac sulfide, on renal cycle-oxygenase. Appropriate clinical use of all nonsteroidal anti-inflammatory drugs, including sulindac, requires careful consideration of risk factors that predispose to nephrotoxicity and careful monitoring when administered to patients at risk.
in vitro
sulindac sulfide was found to be effective at inhibit tumor growth. however, its analog, sulindac sulfone, was reported to exhibit antitumor properties without inhibiting cyclooxygenase activity. previous study investigated the effect of sulindac sulfide or sulfone treatment on the growth of colorectal carcinoma cells. results showed that sulindac sulfide or sulfone treatment of hca-7 cells results in the inhibition of prostaglandin e2 production. moreover, both sulindac sulfide and sulfone could inhibit the in-vitro growth of hca-7 and hct-116 cell lines [1].
in vivo
animal study showed that sulfide derivative inhibited hca-7 in-vivo growth, whereas sulindac sulfone showed no effect on the growth of either hca-7 or hct-116 xenografts [1].
IC 50
1.9 and 1.21 μm for cox-1 and cox-2, respectively
References
1) Duggan et al. (1977) Identification of the biologically active form of sulindac; J. Pharmacol. Exp. Ther., 201 8
2) Herrmann et al. (2000) Sulindac sulfide inhibits Ras signaling; Nature 17 1769
3) Tinsley et al. (2010) Colon tumor cell growth inhibitory activity of sulindac sulfide and other NSAIDS is associated with PDE5 inhibition; Cancer Prev. Res., 3 1303
4) Tinsley et al. (2011) Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin mediated transcription in human breast tumor cells; Cancer Prev .Res., 4 1275
5) Bravi et al. (2015) Sulindac metabolites decrease cerebrovascular malformations in CCM3-knockout mice; Proc. Natl. Acad. Sci. USA, 112 8421
Sulindac sulfide Preparation Products And Raw materials
Raw materials
Preparation Products
Sulindac sulfide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| .GZ HONESTCHEM CO.,LTD | +86-15013270415 | honestchemical@foxmail.com | China | 247 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 | dj@sgtlifesciences.com | China | 12382 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50000 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Enlian Biomedical (Chongqing) Co., Ltd. | 023-63134718 13048300200 | 1501533472@qq.com | China | 60 | 58 |
Related articles
- Sulindac sulfide: mechanism of action, activities and safety
- Sulindac sulfide shows anti-tumorigenic and anti-inflammatory properties but requires careful handling due to safety concerns.
- Dec 4,2023
- NSAIDs——Sulindac Sulfide
- Sulindac sulfide is an aryl sulfide that is a metabolite of sulindac. A non-steroidal anti-inflammatory drug, which also has a....
- Jan 6,2022