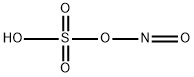
나이트로실황산
|
|
나이트로실황산 속성
- 녹는점
- -10 °C
- 끓는 점
- 333℃ at 101.33kPa
- 밀도
- 1.612 g/mL at 25 °C
- 증기압
- 2.6-30Pa at 20-50℃
- 저장 조건
- Refrigerator (+4°C)
- 용해도
- reacts with H2O; soluble in H2SO4
- 산도 계수 (pKa)
- -7.51±0.18(Predicted)
- 물리적 상태
- 점성 액체
- 색상
- 투명~우유빛 노란색~녹색
- 수용성
- 물에서 분해되다
- Merck
- 13,6682
- InChIKey
- VQTGUFBGYOIUFS-UHFFFAOYSA-N
- CAS 데이터베이스
- 7782-78-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-14-23/24/25-35-34 | ||
| 안전지침서 | 17-26-36/37/39-45-27 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 2 | ||
| WGK 독일 | 3 | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 28111980 | ||
| 기존화학 물질 | KE-26036 |
나이트로실황산 C화학적 특성, 용도, 생산
화학적 성질
Nitrosylsulfuric acid, HNO5S, is a straw-colored, oily liquid furnished as a 40% solution in 87% sulfuric acid,and stable at room temperature. It is used as a diazotizing agent for dyes,chemical intermediate, drugs and pharmaceuticals.물리적 성질
Nitrosylsulfuric acid is produced as an intermediate in the manufacture of sulfuric acid using the lead chamber process by the reaction of sulfur dioxide, nitrogen dioxide, oxygen, and water.Nitrosylsulfuric acid also is made from absorption of nitrogen oxides (NOx) in oleum (fuming sulfuric acid):
N2O3 + 2H2SO4 → 2ONSO4 + H2O
or by the reaction of nitrosyl chloride with concentrated sulfuric acid:
ClNO + H2SO4 → ONSO4 + HCl
Another method of preparation involves the reaction of nitrosyl bromide with silver bisulfate:
AgHSO4 + BrNO → ONHSO4 + AgBr
In making disperse azo dyes, nitrosylsulfuric acid is produced by the addition of sodium nitrite, NaNO2, to concentrated sulfuric acid (1g NaNO2 per 13 g H2SO4).
용도
Nitrosylsulfuric acid is used for diazotisation, nitrosation, oxidation and oximation reactions. Product Data Sheet일반 설명
Shipped in solution with sulfuric acid (solutions are usually 40% Nitrosylsulfuric acid and 54% sulfuric acid (Hawley)). Solution is a straw-colored oily liquid with a sharp odor. When pure, a crystalline solid decomposing at 73°C. Very irritating to skin and eyes. Used to make dyes and other chemicals.반응 프로필
Nitrosylsulfuric acid is an oxidizing agent. Neutralizes chemical bases in exothermic reactions. An attempt to diazotize dinitroaniline using Nitrosylsulfuric acid resulted in an explosion with loss of life. The event was blamed on the high concentration of the reactants dinitroaniline hydrochloride and Nitrosylsulfuric acid [MCA Case History 1763. 1971].위험도
Strong irritant to skin and mucous membranes.건강위험
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Safety Profile
A poison. A corrosive irritant to skin, eyes, and mucous membranes. Explosive reaction above 50°C with 2-chloro-4,6-dinitroaniline and 4- chloro-2,6-d1nitroalne. Potentially explosive reaction with dmitroaniline. When heated to decomposition it emits toxic fumes of SOx and NOx. See also SULFATES and NITRATES.나이트로실황산 준비 용품 및 원자재
원자재
준비 용품
나이트로실황산 공급 업체
글로벌( 117)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12470 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21687 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9338 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 6011 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Jinan Qinmu Fine Chemical Co.,Ltd. | +8618660799346 |
sales@qinmuchem.com | China | 1009 | 58 |
| Nanjing Sinoda Biological Technology Co., Ltd | +8613401983379 |
sales@njmcn.cn | CHINA | 74 | 58 |