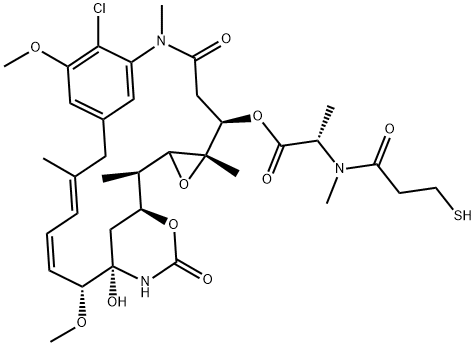
N2'-deacetyl-N2'-(3-Mercapto-1-oxopropyl)-메이탄신
|
|
N2'-deacetyl-N2'-(3-Mercapto-1-oxopropyl)-메이탄신 속성
- 녹는점
- 190-192℃ (decomposition)
- 끓는 점
- 937.1±65.0 °C(Predicted)
- 밀도
- 1.33±0.1 g/cm3(Predicted)
- 저장 조건
- 2-8°C
- 용해도
- DMSO:60.0(Max Conc. mg/mL);81.26(Max Conc. mM)
- 물리적 상태
- 결정성 고체
- 산도 계수 (pKa)
- 9.82±0.70(Predicted)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
N2'-deacetyl-N2'-(3-Mercapto-1-oxopropyl)-메이탄신 C화학적 특성, 용도, 생산
개요
Maytansine, a natural alkaloid, was first isolated from Maydens ovale in 1972 and exists in the genus Maydens of the family Weedaceae and its relatives. The mechanism of action of maytansine, acting on microtubules and tubulin, blocks the formation of spindles in the process of cell mitosis by inhibiting the depolymerization of cell microtubules, and has a strong ability to inhibit tumor cell proliferation.화학적 성질
Mertansine is a white powder. It is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of mertansine in these solvents is approximately 0.25, 20, and 33 mg/ml, respectively.용도
Mertansine (DM1) is a microtubulin inhibitor which binds at the tips of microtubules and suppresses the dynamicity of microtubules.. Mertansine is an antibody-conjugatable maytansinoid that was developed to overcome systemic toxicity associated with maytansine and to enhance tumor-specific delivery. It can be attached to a monoclonal antibody with a linker to create an antibody-drug conjugate (ADC).정의
ChEBI: Mertansine is an organic heterotetracyclic compound and 19-membered macrocyclic lactam that is maytansine in which one of the hydrogens of the terminal N-acetyl group is replaced by a sulfanylmethyl group. It has a role as an antineoplastic agent and a tubulin modulator. It is an alpha-amino acid ester, a carbamate ester, an epoxide, an organic heterotetracyclic compound, an organochlorine compound, a thiol and a maytansinoid. It derives from a maytansine.생물학적 활성
Mertansine is a thiol-containing derivative of maytansine that is cytotoxic to human epidermoid carcinoma KB and human breast cancer SK-BR-3 cells (IC50 = 1.10 nM for both). Formulations containing mertansine have been studied for the treatment of multiple myeloma and squamous cell carcinoma.부작용
Dose-limiting toxicity was observed at 2 mg/m2, and was manifested as profound weakness, diarrhea, nausea, and vomiting.Synthesis
Mertansine synthesis steps: A solution of N 2'-deacetyl-N 2'- (3-methyldithio-1-oxopropyl)- maytansine (2b) (1.95 g, 2.5 mmol) in a mixture of ethyl acetate (140 mL) and methanol (210 mL) was stirred at room temperature under an argon atmosphere and treated with a solution of dithiothreitol (0.95 g, 6.2 mmol) in 0.05 M potassium phosphate buffer (140 mL) at pH 7.5 containing 2 mM ethylenediaminetetraacetic acid (EDTA). The progress of the reaction was monitored by HPLC and was complete in three hours. The reaction mixture was treated with a solution of 0.2 M potassium phosphate buffer (250 mL) at pH 6.0 containing 2 mM EDTA and then extracted with ethyl acetate (3 x 600 mL). The organic layers were combined, washed with brine (100 mL), and then dried over sodium sulfate. Evaporation of the solvent gave a residue of crude thiol-containing maytansinoid 25a. The crude residue was purified by HPLC using a preparative Diazem cyano HPLC column (250 mm x 50 mm, 10 micron particle size) that was equilibrated in a mixture of hexanes/2-propanol/ethyl acetate (78.0:5.5:16.5, v/v/v) and run at a flow rate of 150 mL/min. The desired product 25a eluted as a peak centered at 16 min. The fractions containing the product were evaporated to give mertansine as a white solid (76% yield).
Fig The synthetic method 1 of Mertansine.
Solubility in organics
Mertansine is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF). The solubility of mertansine in these solvents is approximately 0.25, 20, and 33 mg/ml, respectively. Mertansine is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, Mertansine should first be dissolved in DMF and then diluted with the aqueous buffer of choice. Mertansine has a solubility of approximately 0.03 mg/ml in a 1:30 solution of DMF: PBS(pH 7.2)using this method.Mode of action
Mertansine is a 19-member ansa macrolide structure attached to a chlorinated benzene ring. It was originally isolated from the shrub Maytenus ovatus. The antimitotic effect of maytansine has been attributed to its ability to inhibit microtubule assembly by binding to tubulin with a KD of ~1 μmol/L, at or near the vinblastine-binding site. Mertansine is effective in vivo against Lewis lung carcinoma and B16 murine melanocarcinoma solid tumors and has antileukemic activity against P388 murine lymphocytic leukemia.N2'-deacetyl-N2'-(3-Mercapto-1-oxopropyl)-메이탄신 준비 용품 및 원자재
원자재
준비 용품
N2'-deacetyl-N2'-(3-Mercapto-1-oxopropyl)-메이탄신 공급 업체
글로벌( 226)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32686 | 60 |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 |
contact@fuxinpharm.com | China | 10297 | 58 |
| Xilinglab Co., Ltd. | +8618381337976 |
bd@xilinglab.com | China | 86 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15956 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21688 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9341 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 |
sales@fine-chemtech.com | CHINA | 885 | 55 |
| AB PharmaTech,LLC | 323-480-4688 |
United States | 989 | 55 | |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19553 | 58 |
N2'-deacetyl-N2'-(3-Mercapto-1-oxopropyl)-메이탄신 관련 검색:
DM4
MEQUITAZINE
AP3Impurity 2
Maytansinol
Pigment Black 34
GDC-0084
SH-4-54
Osimertinib mesylate
Tazemetostat (EPZ-6438)
Niraparib
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
Enasidenib
Ivosidenib
ABBV-075
Ponatinib
Selonsertib