| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Oxypyrronium bromide;Oxypyrronium bromide
CAS:561-43-3
Purity:98% Package:5 mg
|
|
| | oxypyrronium bromide Basic information |
| Product Name: | oxypyrronium bromide | | Synonyms: | oxypyrronium bromide;Oxypyrronium;OxipyrroniumBromide;(1,1-dimethylpyrrolidin-1-ium-2-yl)methyl 2-cyclohexyl-2-hydroxy-2-phenylacetate bromide;(1,1-dimethylpyrrolidin-1-ium-2-yl)methyl 2-cyclohexyl-2-hydroxy-2-phenyl-ethanoate bromide;2-cyclohexyl-2-hydroxy-2-phenyl-acetic acid (1,1-dimethylpyrrolidin-1-ium-2-yl)methyl ester bromide;Immetropan;L.D. 3055 | | CAS: | 561-43-3 | | MF: | C21H32BrNO3 | | MW: | 426.38768 | | EINECS: | 2092198 | | Product Categories: | | | Mol File: | 561-43-3.mol | 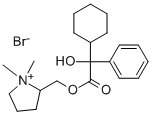 |
| | oxypyrronium bromide Chemical Properties |
| Toxicity | LD50 oral in rat: 780mg/kg |
| | oxypyrronium bromide Usage And Synthesis |
| Originator | Oxypyrronium ,Shanghai Lansheng | | Manufacturing Process | The 1-methyl-2-hydroxymethylpyrrolidine was obtained by the process of Application No 21193/56 (Serial No. 820,503).
A methanolic solution of sodium methoxide [from sodium (0.6 g) and methanol (15 ml)] was added dropwise during 3 h to a boiling solution of methyl phenylcyclohexylglycollate (33.7 g) and 1-methyl-2hydroxymethylpyrrolidine (23.4 g) in heptane (400 ml) and the methanol that separated was removed by means of a Dean and Stark apparatus. At the end of 4 h no further separation of methanol occurred and the solvent was removed under reduced pressure. The residue was dissolved in either and the etheral solution, after washing with water (3 x 50 ml), was extracted with 5 N hydrochloric acid (3 x 100 ml). The (1-methyl-2-pyrrolidyl)methyl phenylcyclokexylglycollate hydrochloride (35.5 g 71%) crystallised out of the acid extract as colourless needles, melting point 181°-196°C. Extraction of this hydrochloride (33.0 g) with hot ethanol (150 ml) left the sparingly soluble (1-methyl-2-pyrrolidyl)methyl phenylcyclokexylglycollate hydrochloride (aform) (7.6 g), melting point 220°-222°C.
The (1-methyl-2-pyrrolidyl)methyl phenylcyclokexylglycollate hydrochloride (aform) (15.0 g) was dissolved in water, basified with sodium hydroxide solution and the resultant oil extracted into ether. The extracts were dried over magnesium sulfate, the ether evaporated and the residue dissolved in acetone (100 ml). Methyl bromide (7.8 g, 2 mole) was added to the acetone solution and the mixture warmed on a steam bath for 15 min. The solution was cooled and the solid filtered off, washed with a little acetone and dried to give the
(1,1-dimethyl-2-pyrrolidyl)methyl α-phenylcyclokexylglycollate bromide, melting point 185°-186°C. (86%). | | Therapeutic Function | Anticholinergic, Spasmolytic |
| | oxypyrronium bromide Preparation Products And Raw materials |
|