- Cyclosporine
-

- $0.00 / 1KG
-
2024-03-16
- CAS:79217-60-0
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
- Cyclosporine
-

- $1.90 / 1KG
-
2023-03-06
- CAS:79217-60-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 ton
- Cyclosporin
-

- $0.00 / 25KG
-
2023-01-31
- CAS:79217-60-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
|
| Product Name: | CYCLOSPORINE | | Synonyms: | CYCLOSPORIN;Cyclosporin A / B / C / D / H A 59865-13-3 /;Cyclosporins;CYCLOSPRINE;CYCLOSPORINE / CICLOSPORIN;Cyclosporine;Cyclosporine (Neoral);Cyclosporine (cyclosporin A) | | CAS: | 79217-60-0 | | MF: | C62H111N11O12 | | MW: | 1202.61 | | EINECS: | 1312995-182-4 | | Product Categories: | Inhibitors;API | | Mol File: | 79217-60-0.mol | 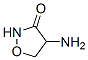 |
| | CYCLOSPORINE Chemical Properties |
| | CYCLOSPORINE Usage And Synthesis |
| Application in Particular Diseases | In Rheumatic Arthritis:
Cyclosporine reduces production of cytokines involved in T-cell activation and has direct effects on B cells, macrophages, bone, and cartilage cells. Its onset appears to be 1 to 3 months. Important toxicities at doses of 1 to 10 mg/kg/day include hypertension, hyperglycemia, nephrotoxicity, tremor, GI intolerance, hirsutism, and gingival hyperplasia. Cyclosporine should be reserved for patients refractory to or intolerant of other DMARDs. It should be avoided in patients with current or past malignancy, uncontrolled hypertension, renal dysfunction, immunodeficiency, low white blood cell or platelet counts, or elevated liver function tests.
| | Description | Cyclosporine is a cyclic polypeptide with potent, partially selective immunosupressive
activity. Isolated from the species Cylindrocarpon lucidium and
Trichoderma polysporum, cyclosporine is useful in the prevention and treatment
of graft/host disease and the prevention of rejection following organ transplantation.
It appears to act by preferentially suppressing T-lymphocytes.
Cyclosporine lacks myelotoxicity, although impaired renal and liver function have
been observed. Initial administration is via the intravenous route, followed by
oral maintenance therapy. | | Chemical Properties | White crystalline solid or powder. | | Originator | Sandoz (Switzerland) | | Indications | Cyclosporine (Sandimmune) is a potent inhibitor of antibody-
and cell-mediated immune responses and is the
immunosuppressant of choice for the prevention of
transplant rejection. It also has application in the treatment
of autoimmune diseases.
Cyclosporine is a highly stable 11-amino acid cyclic
polypeptide. The molecule is very lipophilic and essentially
is not soluble in water. It can be administered intravenously,
orally, or by injection. | | Brand name | SANDIMMUN | | Mechanism of action | Cyclosporine can bind to the cytosolic protein cytophilin
C. This drug–protein complex inhibits calcineurin
phosphatase activity, which leads to a decreased
synthesis and release of several cytokines,
including interleukins IL-2, IL-3, IL-4, interferon-�, and
tumor necrosis factor.
Cyclosporine exhibits a high degree of specificity in
its actions on T cells without significantly impairing Bcell
activity. It can inhibit the T cell–dependent limb of
antibody production by lymphocytes by preventing the
differentiation of B cells into antibody-secreting plasma
cells. Because T cells appear to require IL-2 stimulation
for their continuous growth, cyclosporine impairs the
proliferative response of T cells to antigens. However,
once T cells have been stimulated by antigens to synthesize
IL-2, cyclosporine cannot suppress the proliferation
of T cells induced by this cytokine. | | Pharmacology | After oral administration, cyclosporine is absorbed
slowly and incompletely, with great variation among individuals.
Peak plasma concentrations are reached in
3 to 4 hours, and the plasma half-life is 10 to 27 hours.
The drug is extensively metabolized by hepatic mixedfunction
oxidase enzymes and is excreted principally via
the bile into the feces. Metabolism results in inactivation
of the immunosuppressive activity.Agents that enhance
or inhibit the mixed-function oxidase enzymes
will alter the therapeutic response to cyclosporine. | | Clinical Use | Cyclosporine has been approved for use in allogeneic
kidney, liver, and heart transplant patients and is under
study for use in pancreas, bone marrow, single lung, and
heart–lung transplant procedures. It is recommended
that corticosteroids, such as prednisone, be used concomitantly,
although at half or less of their usual dose.
Such combined therapy leads to fewer side effects, a decreased
incidence of infectious complications, efficacy
of lower doses of cyclosporine, and a better history of
patient survival.
Cyclosporine appears to have promise in the treatment
of autoimmune diseases. It has a beneficial effect
on the course of rheumatoid arthritis, uveitis, insulindependent
diabetes, systemic lupus erythematosus, and
psoriatic arthropathies in some patients. Toxicity is
more of a problem in these conditions than during use
in transplantation, since higher doses of cyclosporine
are often required to suppress autoimmune disorders. | | Side effects | Compared with previously available therapy, the adverse
effects associated with cyclosporine are much less severe
but still worthy of concern. Nephrotoxicity, which can occur
in up to 75% of patients, ranges from severe tubular
necrosis to chronic interstitial nephropathy.This effect is
generally reversible with dosage reduction. Vasoconstriction
appears to be an important aspect of cyclosporine-
induced nephrotoxicity. Hypertension occurs in
25% of the patients and more frequently in patients with
some degree of renal dysfunction; the concomitant use of
antihypertensive drugs may prove useful. Hyperglycemia,
hyperlipidemia, transient liver dysfunction, and
unwanted hair growth are also observed. | | Potential Exposure | Cyclosporin A is a fungal metabolite;
an amide immunosuppressant drug used in various
surgeries. | | Veterinary Drugs and Treatments | Cyclosporine may be useful as an immunosuppressant for immunemediated
diseases
(see dosage section) and as part of a protocol to
reduce the rejection of allografts in transplant medicine in dogs and
cats. | | Shipping | UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | | Incompatibilities | Amides/imides react with azo and diazo
compounds to generate toxic gases. Flammable gases are
formed by the reaction of organic amides/imides with
strong reducing agents such as hydrideds and active metals.
Amides are very weak bases (weaker than water). Imides
are less basic yet and in fact react with strong bases to
form salts. That is, they can react as acids. Mixing amides
with dehydrating agents such as such as phosphorus pent-
oxide or thionyl chloride generates the corresponding
nitrile. The combustion of these compounds generates
mixed oxides of nitrogen (NOx). | | Waste Disposal | t is inappropriate and possibly
dangerous to the environment to dispose of expired or waste
drugs and pharmaceuticals by flushing them down the toilet
or discarding them to the trash. Household quantities of
expired or waste pharmaceuticals may be mixed with wet
cat litter or coffee grounds, double-bagged in plastic, discard
in trash. Larger quantities shall carefully take into consider-
ation applicable DEA, EPA, and FDA regulations. If possi-
ble return the pharmaceutical to the manufacturer for proper
disposal being careful to properly label and securely package
the material. Alternatively, the waste pharmaceutical shall be
labeled, securely packaged, and transported by a state
licensed medical waste contractor to dispose by burial in a
licensed hazardous or toxic waste landfill or incinerator. |
| | CYCLOSPORINE Preparation Products And Raw materials |
|