- 4-Acryloylmorpholine
-

- $0.00 / 200kg
-
2024-05-23
- CAS:5117-12-4
- Min. Order: 20kg
- Purity: 98.0%
- Supply Ability: 10 tons
- 4-Acryloylmorpholine
-

- $78.00 / 1kg
-
2024-05-20
- CAS:5117-12-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- 4-Acryloylmorpholine
-

- $1.00 / 1g
-
2024-04-25
- CAS:5117-12-4
- Min. Order: 1g
- Purity: 99
- Supply Ability: 20tons
|
| | 4-Acryloylmorpholine Basic information |
| Product Name: | 4-Acryloylmorpholine | | Synonyms: | 4-ACRYLOYLMORPHOLINE;4-(1-oxo-2-propenyl)-Morpholine;N-Acrolylmorpholine;ACRYOYL MORPHOLINE;4-Acryloylmorpholine (stabilized with MEHQ);Morpholine, 4-(1-oxo-2-propenyl)-;Acryloylmorpholin;4-ACRYLOYLMORPHOLINE, 98+%, STAB. WITH 4-METHOXYPHENOL | | CAS: | 5117-12-4 | | MF: | C7H11NO2 | | MW: | 141.17 | | EINECS: | 418-140-1 | | Product Categories: | Acrylate;Acrylic Monomers;Monomers | | Mol File: | 5117-12-4.mol | 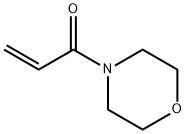 |
| | 4-Acryloylmorpholine Chemical Properties |
| Melting point | −35 °C(lit.) | | Boiling point | 158°C 50mm | | density | 1.122 g/mL at 25 °C(lit.) | | vapor pressure | 1.03-1.64Pa at 25-29.9℃ | | refractive index | n20/D 1.512(lit.) | | Fp | >230 °F | | storage temp. | 2-8°C | | solubility | Soluble in water | | form | Liquid | | pka | -1.08±0.20(Predicted) | | color | Colorless to yellow | | Water Solubility | 1000g/L at 20℃ | | Sensitive | Light Sensitive | | BRN | 119302 | | InChIKey | XLPJNCYCZORXHG-UHFFFAOYSA-N | | LogP | -0.46 at 20.5-21℃ | | CAS DataBase Reference | 5117-12-4(CAS DataBase Reference) | | NIST Chemistry Reference | N-acryloylmorpholine(5117-12-4) | | EPA Substance Registry System | Morpholine, 4-(1-oxo-2-propenyl)- (5117-12-4) |
| | 4-Acryloylmorpholine Usage And Synthesis |
| Chemical Properties | 4-Acryloylmorpholine has a melting point of ?35 °C and is clear liquid at room
temperature. At 25 °C, it has a density of 1.122 g/mL. It needs to be
stored at 2-8°C. | | Uses | 4-Acryloylmorpholine is used in adhesives, UV curable resins, industrial coatings, UV printing ink, oil recovery polymer, medicinal and commodity chemicals. | | Reactions | Using cobalt catalysis, diethylzinc promotes the conjugate reduction of 4-acryloylmorpholine to produce the corresponding ethylzinc enolate, which reacts with N-tosyl aldimines to afford β-aminoamides[1].
 | | Flammability and Explosibility | Non flammable | | Synthesis | A
solution of 0.04 mol of the corresponding amine in 20 ml of anhydrous
methylene chloride was slowly added at 0-5??C to 0.02 mol of acryloyl
chloride in 20 ml of anhydrous methylene chloride. The mixture was
stirred for 3 h at room temperature in an inert atmosphere, and the
precipitate was filtered off and washed with methylene chloride (2 ?á 10
ml). The organic layer was washed in succession with 5 ml of water and 5
ml of a saturated solution of NaHCO3 and dried over Na2SO4, the solvent
was removed under reduced pressure, and the residue was purified by
column chromatography on silica gel using hexane-ethyl acetate (5 : 1 to
1 : 1) as eluent. 4-Acryloylmorpholine, Yield 1.78 g (63%).
IR spectrum, |í, cm-1: 2857, 1647, 1612, 1439, 1263, 1238, 1115, 1038,
953. 1H NMR spectrum, |?, ppm: 3.51-3.73 m (8H, NCH2CH2O),
5.72 d.d (1H, 3-Hcis, 3J = 10.6, 2J = 1.9 Hz), 6.29 d.d (1H, 3-Htrans,
3J = 16.7, 2J = 1.9 Hz), 6.57 d.d (1H, 2-H, J = 16.7, 10.6 Hz). 13C NMR
spectrum, |?C, ppm: 41.74 and 45.66 (CH2N), 66.22 (CH2O), 126.64 (C2),
127.69 (C3), 164.92 (C1). Mass spectrum, m/z (Irel, %): 141 (36) [M]+,
140 (12), 126 (58), 112 (22), 111 (15), 110 (15), 109 (12), 98 (10), 96
(26), 86 (72), 83 (13), 70 (14), 68 (14), 57 (17), 56 (86), 55 (100),
42 (23).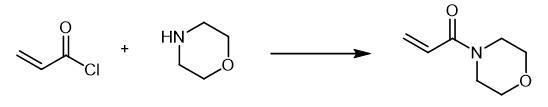 Fig. The synthetic method 2 of 4-Acryloylmorpholine Fig. The synthetic method 2 of 4-Acryloylmorpholine | | References | [1] PRIETO O, LAM H W. Cobalt-catalyzed reductive Mannich reactions of 4-acryloylmorpholine with N-tosyl aldimines??[J]. Organic & Biomolecular Chemistry, 2007. DOI:10.1039/B715839D. |
| | 4-Acryloylmorpholine Preparation Products And Raw materials |
|