- Retapamulin
-
- $0.00 / 25kg
-
2023-11-03
- CAS:224452-66-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2 t
- RETAPAMULIN
-

- $60.00 / 1kg
-
2023-03-24
- CAS:224452-66-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 20 tons
- RETAPAMULIN
-

- $0.00 / 1kg
-
2022-09-08
- CAS:224452-66-8
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
|
| | RETAPAMULIN Basic information |
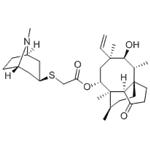
| Product Name: | RETAPAMULIN | | Synonyms: | RETAPAMULIN;RetapaMulin (SB-275833);(3aS,4R,5S,6S,8R,9R,9aR,10R)-2-(exo-8-Methyl-8-azabicyclo[3.2.1]octan-3-ylsulfanyl)acetic acid 5-hydroxy-4,6,9,10-tetraMethyl-1-oxo-6-vinylperhydro-3a,9-propanocyclopentacycloocten-8-yl ester;RetapaMulin API;tetapaMulin;(1S,2R,3S,4S,6R,7R,8R,14R)-4-ethenyl-3-hydroxy-2,4,7,14-tetraMethyl-9-oxotricyclo[5.4.3.0^{1,8}]tetradecan-6-yl 2-{[(1R,3S,5S)-8-Methyl-8-azabicyclo[3.2.1]octan-3-yl]sulfanyl}acetate;Rebapamulin;SB-275833 | | CAS: | 224452-66-8 | | MF: | C30H47NO4S | | MW: | 517.76 | | EINECS: | 639-491-7 | | Product Categories: | Inhibitors;API, Antibiotics | | Mol File: | 224452-66-8.mol |  |
| | RETAPAMULIN Chemical Properties |
| Boiling point | 594.9±50.0 °C(Predicted) | | density | 1.16±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | insoluble in H2O; ≥114.8 mg/mL in EtOH; ≥16.15 mg/mL in DMSO | | form | solid | | pka | 14.65±0.70(Predicted) | | InChIKey | STZYTFJPGGDRJD-IFPFAXJDNA-N | | CAS DataBase Reference | 224452-66-8(CAS DataBase Reference) |
| | RETAPAMULIN Usage And Synthesis |
| Description | Antibacterial retapamulin is a derivative of the natural
product pleuromutilin and was developed by Glaxo and approved
in the US in 2007 for the treatment of skin infections. It has a unique mechanism of action, inhibiting bacterial
protein synthesis by inhibiting the larger subunit of the ribosome,
and thus has no cross resistance to other antibacterial
agents.
Pleuromutilin is a tricyclic diterpenoid that was first isolated in 1951 from the edible mushroom Pleurotus mutilus. The first semisynthetic analogs tiamulin and valnemulin, developed for veterinary use, have been shown to interact uniquely with bacterial ribosomes by high affinity binding to a site on the 50S subunit. Binding to this site interferes with ribosomal peptidyl transferase activity, blocks P-site interactions, and prevents the evolution of active 50S ribosomal subunits. Retapamulin, the first pleuromutilin approved for human use, behaves similarly to selectively inhibit bacterial protein synthesis. This novel mechanism of action has been implicated in the lack of in vitro target-specific cross-resistance with other classes of antibiotics. | | Originator | GlaxoSmithKline (US) | | Uses | A broad spectrum antibiotic with no cross resistance to existing classes | | Uses | Retapamulin is a semi-synthetic pleuromutilin prepared by reacting pleuromutilin tosylate with tropine-3-thiol to give a more hydrophobic analogue with a tertiary amine. This enables formulation as a stable hydrochloride salt. Retapamulin is a broad spectrum antibiotic with no cross resistance to existing antibiotic classes, and is the first pleuromutilin approved for human use. Like all the pleuromutilins, retapamulin inhibits protein synthesis by binding to domain V of 23S rRNA. | | Uses | Retapamulin is a topical antibiotic, which binds to both E. coli and S. aureus ribosomes with similar potencies with Kd of 3 nM | | Uses | Retapamulin is a semi-synthetic pleuormutilin prepared by reacting pleuromutilin tosylate with tropine-3-thiol to give a more hydrophobic analogue with a tertiary amine. This enables formulation as a stable hydrochloride salt. Retapamulin is a broad spectrum antibiotic with no cross resistance to existing antibiotic classes, and is the first pleuromutilin approved for human use. Like all the pleuromutilins, retapamulin inhibits protein synthesis by binding to domain V of 23S rRNA. | | Definition | ChEBI: Retapamulin is a carbotricyclic compound, a carboxylic ester and a cyclic ketone. | | Brand name | Altabax | | Pharmaceutical Applications | A semisynthetic pleuromutilin formulated as a 1% ointment
for topical use. It is active against staphylococci
(MIC 0.12 mg/L), including methicillin-resistant strains, and
against streptococci (MIC 0.03–0.25 mg/L), including Str.
pyogenes and Str. pneumoniae. Most enterococci and Gramnegative
bacilli are resistant. Propionibacteria are susceptible,
suggesting that it might be useful in acne. Early indications
suggest that resistance does not emerge readily, but experience
with veterinary pleuromutilins indicates that chromosomal
resistance may develop with extended use.
It is metabolized in the liver and rapidly excreted, precluding
use in systemic infection. Systemic exposure is said to be
low following topical application and it appears safe, but there
are few data on absorption through broken and unbroken skin.
Principal side effects noted include local irritation and occasional
allergic reactions. Licensed use is presently restricted to
the treatment of impetigo and uncomplicated skin infections.
Possible value in methicillin-resistant Staph. aureus (MRSA)
infection or carriage has not yet been established. | | Side effects | The most frequent adverse event was application site irritation, but other side effects, occurring in o2% of patients, included headache, diarrhea, nausea, and nasopharyngitis. While there are no contraindications, it is recommended that pregnant women only use retapamulin when the potential benefits outweigh the potential risks since animal reproductive studies are not always predictive of human response. Likewise, nursing mothers are cautioned about the unknown possibility of retapamulin excretion in breast milk. | | Synthesis | The synthesis of retapamulin begins with generation of the mesylate of pleuromutilin, isolated through fermentation of Clitopilus passeckerianus, followed by nucleophilic substitution with exo-8-methyl-8-azabicyclo[3.2.1]octan-3-thiol under basic conditions Shridhar Hegde and Michelle Schmidt (potassium-tert-butoxide in ethanol or tetrabutylammonium hydrogen sulfate in dichloromethane/water and sodium hydroxide at pH 12.5). The azabicyclic thiol derivative may be prepared via a Mitsunobu reaction between tropine and thioacetic acid. | | references | [1] jones r n, fritsche t r, sader h s, et al. activity of retapamulin (sb-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. antimicrobial agents and chemotherapy, 2006, 50(7): 2583-2586.
[2] rittenhouse s, biswas s, broskey j, et al. selection of retapamulin, a novel pleuromutilin for topical use. antimicrobial agents and chemotherapy, 2006, 50(11): 3882-3885. |
| | RETAPAMULIN Preparation Products And Raw materials |
| Raw materials | 8-Azabicyclo[3.2.1]octane-3-thiol, 8-Methyl-, (3-exo)--->2-[(Methylsulfonyl)oxy]acetic acid (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester-->Tropine-3-mesylate-->TiaMulin Related CoMpound A-->Tropine |
|