| | MERCURIC NITRATE Basic information |
| Product Name: | MERCURIC NITRATE | | Synonyms: | nitratemercurique;nitratemercurique(french);Nitricacid,mercury(2+)salt;nitricacid,mercury(2++)salt;nitricacid,mercury(ii)salt;Mercury(II)nitrate0,005mol/l(0,01N)DC;MERCURIC(II)NITRATE;Quecksilber(II)-nitrat | | CAS: | 10045-94-0 | | MF: | HgN2O6 | | MW: | 324.6 | | EINECS: | 233-152-3 | | Product Categories: | Inorganics | | Mol File: | 10045-94-0.mol | 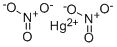 |
| | MERCURIC NITRATE Chemical Properties |
| Melting point | 79°C | | Boiling point | decomposes [CRC10] | | density | 1.025 g/mL at 25 °C | | solubility | soluble in H2O; insoluble in ethanol | | form | colorless hygroscopic crystals | | color | colorless hygroscopic crystals, crystalline | | Water Solubility | soluble H2O; insoluble EtOH [CRC10] | | Stability: | Stable. Incompatible with strong reducing agents, combustible materials, most common metals. | | CAS DataBase Reference | 10045-94-0(CAS DataBase Reference) | | EPA Substance Registry System | Mercuric nitrate (10045-94-0) |
| | MERCURIC NITRATE Usage And Synthesis |
| Description | Mercuric Nitrate is a white to yellowish crystalline solid with a nitric acid-like odor. Normally exists as hemihydrate or dihydrate. Molecular weight= 324.61;Boiling point=(decomposes); Freezing/Melting point=7079℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity 0. Soluble in water. | | Chemical Properties | white to yellow crystalline powder with nitric acid odour | | Chemical Properties | Mercuric nitrate is a white to yellowish crystalline solid with an odor like nitric acid. Normally exists
as the hemihydrate or the dihydrate | | Uses | Nitration of aromatic organic compounds, felt
manufacture, mercury fulminate manufacturing. | | General Description | A white crystalline solid. Toxic by inhalation, ingestion and/or skin contact. Prolonged exposure to fire or heat may result in an explosion. Produces toxic oxides of nitrogen when heated to decomposition. Used to make other chemicals and in medicine. | | Air & Water Reactions | Deliquescent. Soluble in a small amount of water. With much water or on boiling with water, an insoluble basic salt is formed. | | Reactivity Profile | MERCURIC NITRATE is noncombustible, but, as an oxidizing agent, will accelerate the burning of combustible materials. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result. Light sensitive. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. Acetylene forms a sensitive acetylide when passed into an aqueous solution of MERCURIC NITRATE [Mellor 4:933. 1946-47]. Should not be mixed with alcohols as explosive mercury fulminates may be formed [Bahme 1961. p. 9]. Is violently reduced by hypophosphoric acid [Mellor 4:993. 1946-47]. Reacts with phosphine to give a yellow precipitate that explodes when heated or subjected to shock [Mellor 4:993. 1946-47]. | | Hazard | Dangerous fire risk in contact with organic
materials. Very toxic. | | Health Hazard | Acute systemic poisoning may be fatal within a few minutes; death by uremic poisoning is usually delayed 5-12 days. Acute poisoning has resulted from inhaling dust concentrations of 1.2-8.5 mg/m 3 of air; symptoms inc lude tightness and pain in chest, coughing, and difficulty in breathing. Ingestion causes necrosis, pain, vomiting, and severe purging. Contact with eyes causes ulceration of conjunctiva and cornea. Contact with skin causes irritation and po ssible dermatitis; systemic poisoning can occur by absorption through skin. | | Safety Profile | Poison by ingestion,
skin contact, intraperitoneal, and
subcutaneous routes. A powerful oxidizer.
Probably an eye, skin, and mucous
membrane irritant. Reacts with acetylene to form the explosive mercury acetylide whch
is sensitive to heat, friction, or contact with
sulfuric acid. Reaction with ethanol forms
the explosive mercury fulrmnate. Reaction
with isobutene forms an unstable explosive
product. Forms explosive mixtures with
phosphine (heatand impact-sensitive),
potassium cyanide (heat-sensitive), and
sulfur. Violent reaction with phosphinic
acid, hypophosphoric acid, unsaturated
hydrocarbons, aromatics. Vigorous reaction
with petroleum hydrocarbons. When heated
to decomposition it emits very toxic fumes
of Hg and NOx. See also MERCURY
COMPOUNDS, INORGANIC; and
NITRATES. | | Potential Exposure | Mercuric nitrate is used in making
other chemicals; in felt manufacture and in making mercury
fulminate | | First aid | If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Antidotes and special procedures for medical personnel: The drug NAP (n-acetyl penicillamine) has been used to treat mercury poisoning, with mixed success. Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has been used to treat toxic symptoms of certain heavy metals poisoning including mercury. Although BAL is reported to have a large margin of safety, caution must be exercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with 1-ephedrine sulfate (CAS: 134-72-5). | | storage | Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Mercuric nitrate must be stored to avoid contact with organic materials; acetylene, ethanol, phosphine, sulfur, and hypophosphoric acid, since violent reactions occur. See also “Incompatibilities.” Do not store on wooden floors. | | Shipping | UN1625 Mercuric nitrate, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials. | | Incompatibilities | A strong oxidizer. Reacts violently with
combustibles, petroleum hydrocarbons; reducing agents;
aldehydes, ammonia, ketones, phosphorus. Reacts with
acetylene, alcohol, phosphine, and sulfur to form shocksensitive compounds. Aqueous solution attacks most
metals. Vigorous and dangerous reaction with petroleum
hydrocarbons. Incompatible with organic materials;
acetylene, ethanol, phosphine, sulfur, hypophosphoric acid.
Inorganic mercury compounds are incompatible with acetylene, ammonia, chlorine dioxide; azides, calcium (amalgam
formation), sodium carbide; lithium, rubidium, copper.
Decomposes in heat or on exposure to light, producing
toxic fumes (mercury, nitrogen oxides) |
| | MERCURIC NITRATE Preparation Products And Raw materials |
|