| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:CyclopentaMine Hydrochloride
CAS:538-02-3
Package:250Mg,25Mg
|
| Company Name: |
Chemsky (shanghai) International Co.,Ltd
|
| Tel: |
021-50135380 |
| Email: |
shchemsky@sina.com |
| Products Intro: |
Product Name:CyclopentaMine Hydrochloride;N,α-DiMethylcyclopentaneethanaMine Hydrochloride; N,α-DiMethylcyclopentaneethylaMine Hydrochloride; Clopane Hydrochloride; Cyclonarol Hydrochloride; Cyclopentadrin Hydrochloride; Cyclopentadrine Hydrochloride; CyclopentaMin Hy
CAS:538-02-3
Purity:98+% Package:1Mg;5Mg;10Mg;50Mg;100Mg;500Mg
|
| Company Name: |
Chembon Pharmaceutical Co., Ltd.
|
| Tel: |
028-84252981 |
| Email: |
sales@chembon.com.cn |
| Products Intro: |
Product Name:cyclopentaMine hydrochloride
CAS:538-02-3
Purity:>97% Package:5g,25g,100g,500g,1kg,10kg
|
| Company Name: |
BOC Sciences
|
| Tel: |
16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Cyclopentamine Hydrochloride
CAS:538-02-3
Purity:95%
|
|
| | cyclopentamine hydrochloride Basic information |
| Product Name: | cyclopentamine hydrochloride | | Synonyms: | Cyclopentadrin Hydrochloride;Cyclopentadrine Hydrochloride;CyclopentaMin Hy;N,α-DiMethylcyclopentaneethanaMine Hydrochloride;N,α-DiMethylcyclopentaneethylaMine Hydrochloride;cyclopentamine hydrochloride;(2-cyclopentyl-1-methyl-ethyl)-methyl-amine hydrochloride;1-cyclopentyl-N-methyl-propan-2-amine hydrochloride | | CAS: | 538-02-3 | | MF: | C9H20ClN | | MW: | 177.7148 | | EINECS: | 2086818 | | Product Categories: | | | Mol File: | 538-02-3.mol | 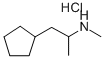 |
| | cyclopentamine hydrochloride Chemical Properties |
| Melting point | 114.5°C | | Boiling point | 292.98°C (rough estimate) | | density | 0.9875 (rough estimate) | | refractive index | 1.6224 (estimate) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Off-White |
| | cyclopentamine hydrochloride Usage And Synthesis |
| Originator | Clopane,Lilly,US,1951 | | Uses | Cyclopentamine is a sympathomimetic alkylamine. Cyclopentamine is a vasoconstrictor that acts as a releasing agent of the neurotransmitters norepinephrine, epinephrine and dopamine. | | Manufacturing Process | A mixture of 126 g (1.5 mols) of cyclopentanone, 128 g (1.5 mols)
cyanoacetic acid, 31 g (0.5 mol) of ammonium acetate and 200 cc of dry
benzene is heated under a refluxing condenser and a water trap. The mixture
is refluxed for about 12 hours after which time no more water collects in the
trap, and the formation of cyclopentylideneacetonitrile is complete. The
reaction mixture comprising a mixture of cyclopentylideneacetonitrile and
cyclopentylideneacetic acid is washed with about one liter of 2% hydrochloric
acid and the benzene layer is separated and the mixture is distilled to cause
decarboxylation of the cyclopentylideneacetic acid present. The distillate
comprising cyclopentylideneacetonitrile which boils at 172° to 175°C is
purified by distillation.
A mixture of 53.5 g (0.5 mol) of cyclopentylideneacetonitrile dissolved in 50
cc of absolute ethanol and 0.5 g of a palladium-carbon catalyst is
hydrogenated with hydrogen at a pressure of about 40 lb for about 3 hours.
An additional amount of 0.8 g of palladium-carbon catalyst is then added and
the hydrogenation continued for about 4 hours during which time the
reduction is substantially completed and the cyclopentylideneacetonitrile is
converted to cyclopentylacetonitrile. The reaction mixture is filtered to remove
the catalyst and the alcohol is evaporated in vacuo.
The residue comprising chiefly cyclopentylacetonitrile is washed with dilute
hydrochloric acid to remove any amine which may have been formed during
the hydrogenation process, and the organic residue comprising
cyclopentylacetonitrile is dissolved in ether, the ether solution dried over
anhydrous magnesium sulfate and distilled. The cyclopentylacetonitrile boils at
185° to 187°C and has a refractive index of nD25 = 1.4456.
To an ethereal solution of methyl magnesium iodide prepared from 26.7 g (1.1
mols) of magnesium and 160 g (1.13 mols) of methyl iodide in 200 cc of dry
ether, is added a solution of 79 g (0.72 mol) of cyclopentylacetonitrile in 100
cc of dry ether. The reaction mixture is refluxed for 4 hours. The reaction
mixture is then decomposed with ice in the usual way, and the ether layer containing the cyclopentylacetone is separated, is dried over anhydrous
magnesium sulfate and the ether removed by evaporation. The residue
comprising cyclopentylacetone is purified by distillation in vacuo. The
cyclopentylacetone boils at 82° to 84°C at about 32 mm pressure.
A mixture of 75 g (0.6 mol) of cyclopentylacetone, 75 g (2.4 mols) of
methylamine, and 10 g of Raney nickel catalyst is placed in a high pressure
bomb previously cooled to a temperature below -6°C, and hydrogen is
admitted under an initial pressure of about 2,000 psi. The bomb is then
heated to about 135° to 150°C for about 2 hours, during which time reductive
amination takes place and 1-cyclopentyl-2-methylaminopropane is produced.
During the period of heating the reaction mixture is agitated by rocking the
bomb. The bomb is then cooled and opened thus permitting the escape of
hydrogen and most of the excess methylamine. The reaction mixture is
filtered to remove the nickel catalyst and the filtrate comprising 1-cyclopentyl-
2-methylaminopropane is purified by distillation under reduced pressure. 1-
Cyclopentyl-2-methylaminopropane boils at 83° to 86°C at about 30 mm
pressure.
1-Cyclopentyl-2-methylaminopropane thus produced is a colorless liquid of
slightly ammoniacal odor. It has a refractive of nD25 = 1.4500. Analysis
showed the presence of 9.79% N as compared with a calculated value of
9.99% N.
141 g (1 mol) of 1-cyclopentyl-2-methylaminopropane are dissolved in 500 cc
of dry ether, and dry hydrogen chloride is passed into the solution until the
weight of the mixture and container has increased by 36 g. During the
addition of the hydrogen chloride, the hydrochloric acid addition salt of 1-
cyclopentyl-2-methylaminopropane precipitates as a white powder. The salt is
filtered off and washed with dry ether. 1-Cyclopentyl-2-methylaminopropane
hydrochloride thus prepared melts at about 113° to 115°C. The yield is
practically quantitative. | | Brand name | Clopane Hydrochloride
(Lilly). | | Therapeutic Function | Vasoconstrictor |
| | cyclopentamine hydrochloride Preparation Products And Raw materials |
|