- Abemaciclib
-

- $0.00 / 100g
-
2024-05-10
- CAS:1231929-97-7
- Min. Order: 50g
- Purity: 99%HPLC
- Supply Ability: 5Kg
- Abemaciclib
-

- $0.00 / 1g
-
2024-03-12
- CAS:1231929-97-7
- Min. Order: 1g
- Purity: 98% HPLC
- Supply Ability: 1kg
- Abemaciclib
-

- $0.00 / 1kg
-
2023-10-11
- CAS:1231929-97-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons
|
| | Abemaciclib Basic information |
| | Abemaciclib Chemical Properties |
| Boiling point | 689.3±65.0 °C(Predicted) | | density | 1.32±0.1 g/cm3(Predicted) | | storage temp. | 4°C, protect from light | | solubility | insoluble in H2O; ≥4.83 mg/mL in DMSO with gentle warming and ultrasonic; ≥6.34 mg/mL in EtOH with gentle warming | | form | solid | | pka | 7.69±0.10(Predicted) | | InChIKey | UZWDCWONPYILKI-UHFFFAOYSA-N | | SMILES | C1(NC2=NC=C(CN3CCN(CC)CC3)C=C2)=NC=C(F)C(C2C=C3N(C(C)C)C(C)=NC3=C(F)C=2)=N1 |
| | Abemaciclib Usage And Synthesis |
| Characteristics |
Class: serine/threonine protein kinase
Treatment: Breast cancer
Oral bioavailability = 46%
Elimination half-life = 18 h
Protein binding = 95–98%
| | Uses | Abemaciclib is a potent and selective inhibitor of cyclin-dependent kinases, CDK4 and CDK6, as a method to inhibit the proliferation of cancer cells. | | Indications | Abemaciclib is used to treat breast cancer that is estrogen receptor-positive (ER-positive) and HER2-negative. It can be taken by both men and women. Patients may be offered abemaciclib if they have:
Early primary breast cancer that the treatment team thinks has a higher risk of coming back (recurrence). Abemaciclib may be provided as an adjuvant treatment, meaning a treatment given after initial treatment, such as surgery;
Breast cancer that has spread to the tissues and lymph nodes around the chest, neck, and under the breastbone (locally advanced breast cancer);
Breast cancer has spread to other parts of the body, such as the bones, lungs, liver, or brain (secondary breast cancer). | | Biological Activity | ly2835219 is an orally available cyclin-dependent kinase (cdk) inhibitor that targets the cdk4 (cyclin d1) and cdk6 (cyclin d3) cell cycle pathway, with potential antineoplastic activity. cdk4/6 dual inhibitor ly2835219 specifically inhibits cdk4 and 6, thereby inhibiting retinoblastoma (rb) protein phosphorylation in early g1. inhibition of rb phosphorylation prevents cdk-mediated g1-s phase transition, thereby arresting the cell cycle in the g1 phase, suppressing dna synthesis and inhibiting cancer cell growth. overexpression of the serine/threonine kinases cdk4/6, as seen in certain types of cancer, causes cell cycle deregulation. | | Biochem/physiol Actions | LY2835219 (abemaciclib) was identified via compound and biochemical screening by scientists at Eli Lilly and Company Research Laboratories and selected for its biological activity and highly selective inhibition of the complexes CDK4/ cyclin D1 (IC50 =2 nmol/L) and CDK6/cyclin D1 (IC50 =10 nmol/L), with no activity against other CDK/cyclin complexes or cell-cycle-related kinases within the nanomolar ranges, except for inhibition of CDK9 at IC50 at least five times higher. The compound was shown to act as a competitive inhibitor of the ATP-binding domain of the CDK4 and CDK6 and to be 14 times more potent against CDK4 than against CDK6. In comparison to palbociclib and ribociclib, abemaciclib shows higher selectivity for the complex CDK4/cyclin D1, with IC50 values five times lower than those of the two other compounds[1]. | | Synthesis |
A concise total synthesis of CDK 4/6 inhibitor abemaciclib is described below. The synthesis uses a Suzuki coupling and a Hartwig–Buchwald amination to join three of the four subunits. The final step is a reductive amination utilizing Leuckart–Wallach conditions. Key to the Leuckart–Wallach reaction was the addition of trimethyl orthoformate to remove water formed during the reaction, allowing the reaction to complete[4].
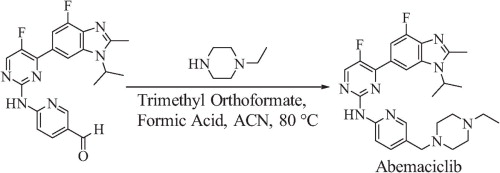
| | Enzyme inhibitor | This oral cell cycle inhibitor (FWfree-base = 506.61 g/mol; FWmesylate-salt =
602.70 g/mol; CAS 1231930-82-7 (mesylate salt) ), also known as
LY2835219 and N-[5-[ (4-ethyl-1-piperazinyl) methyl]-2-pyridinyl]-5-
fluoro-4-[4-fluoro-2-methyl-1- (1-methylethyl) -1H-benzimidazol-6-yl]-2-
pyrimidinamine, targets the cyclin-dependent kinase CDK4, or cyclin D1
(IC50 = 2 nM) and CDK6, or cyclin D3 (IC50 = 6 nM), inhibiting
retinoblastoma (Rb) protein phosphorylation in early G1, thereby arresting
the cell cycle in the G1, suppressing DNA synthesis, and inhibiting cancer
cell growth. LY2835219 inhibits activation of AKT and ERK, but not
mTOR. | | References |
[1] Silvia Paola Corona, Daniele Generali. “Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer.” Drug Design, Development and Therapy (2018): 321–330.
[2] “OS Trending Positive for Abemaciclib.” Cancer discovery 13 1 (2023): OF3.
[3] Erin R Scheidemann. “Resistance to abemaciclib is associated with increased metastatic potential and lysosomal protein deregulation in breast cancer cells.” Molecular Carcinogenesis (2024): 209–223.
[4] Michael O. Frederick, Douglas P. Kjell. “A synthesis of abemaciclib utilizing a Leuckart–Wallach reaction.” Tetrahedron Letters 56 7 (2015): Pages 949-951.
|
| | Abemaciclib Preparation Products And Raw materials |
| Raw materials | EthaniMidaMide, N-(4-broMo-2,6-difluorophenyl)-N'-(1-Methylethyl)--->4-Bromo-2,6-difluoroaniline-->2-Bromopyridine-5-carbaldehyde-->Piperazine, 1-[(6-broMo-3-pyridinyl)Methyl]-4-ethyl--->N-Isopropylacetamide-->1H-BenziMidazole, 6-(2-chloro-5-fluoro-4-pyriMidinyl)-4-fluoro-2-Methyl-1-(1-Methylethyl)--->1H-BenziMidazole, 4-fluoro-2-Methyl-1-(1-Methylethyl)-6-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)--->4-AMINO-3,5-DIFLUOROACETOPHENONE-->5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-amine-->6-Bromo-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole-->1-Ethylpiperazine-->2,6-Difluoroaniline |
|