The use of Mitomycin C in the treatment of bladder cancer
Apr 12,2024
Description
Bladder cancer (BC) is the 10th most common cancer worldwide, with approximately 573,000 new cases and 213,000 associated deaths reported in 2020. At presentation, approximately 70%-75% of patients have nonmuscle-invasive BC (NMIBC) confined to the mucosa (stage Ta, carcinoma in situ [CIS]) or submucosa (stage T1) of the bladder with an intrinsic risk of recurrence and progression to muscle-invasive BC (MIBC). The standard therapy for NMIBC is complete transurethral resection of bladder tumor (TURBT) followed by intravesical immuno-chemotherapy based on the risk factors. The probability of recurrence at 1 and 5 years after TURBT ranges from 15% to 61% and 31% to 78%, respectively.

NMIBC recurrence occurs via 4 mechanisms—incomplete resection, tumor cell reimplantation, microscopic tumor growth, and new tumor formation. Immediate single-dose intravesical chemotherapy, preferably within 4 hours after TURBT, provides a 15% absolute risk reduction in tumor recurrence by destroying floating tumor cells following TURBT in addition to having an ablative effect on residual tumor cells and the small residual unnoticed tumors after TURBT.
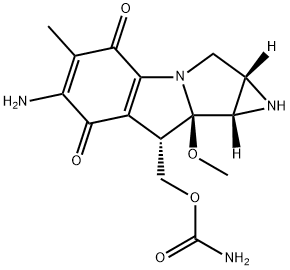
Mitomycin C
Mitomycin C (MMC) is an alkylating agent that causes DNA damage, including interstrand cross-links and single- or double-strand breaks, thereby blocking DNA replication and transcription. It is an antitumor antibiotic that inhibits DNA synthesis by producing DNA cross-links, which halt cell replication and eventually cause cell death. Since cancer cells generally divide faster and with less error-correcting than healthy cells, they are more sensitive to this damage. This cell damage slows or stops the growth of cancer cells in your body. Mitomycin is a chemotherapy drug that aims to kill any cancer cells that may have remained in your bladder after surgery. The drug itself stays in your bladder for one to two hours and is then drained out through the urinary catheter or leaves your bladder when you pass urine[1].
It may have a better tolerated systemic toxicity profile than other intravesical agents because of its high molecular weight and hydrophobicity, which lead to poor systemic absorption across the urothelium. However, it is essential not to ignore the potential toxicities associated with extravasation and systemic absorption, as MMC is a representative vesicant chemotherapeutic agent with deep-tissue penetration and necrotizing properties.
Survival rate
242 patients were treated with weekly instillations of 40 mg Mitomycin C for 8 weeks after transurethral resection. Tumor-free patients then followed a maintenance course with monthly instillations for 3 months. Overall, patients have been followed for a median time of 57 (range 10-114) months. During this period, the recurrence rate was 4.9. Eleven more patients had disease progression. The progression rate is 5.8% (14/242), with a mean time to progression of 34 months. At present, 209 patients are alive; 6 have died of bladder cancer, 16 of causes unrelated to the disease, and 11 (4.5%) have been lost to follow-up. Thus, the crude survival rate is 86.4%, disease-specific mortality is 2.5%, and non-disease-specific mortality is 6.6%. Hence, patients with multiple tumors seem to benefit the least from MMC instillation. Probably, recurrent disease could be better prevented with intravesical bacillus Calmette-Guérin[2].
Side effects
Mitomycin can cause skin irritation if it comes into contact with the skin. Washing the area with soap and water after urinating can reduce this risk.
Some patients will develop a bladder infection after this procedure. If you experience an urgency to urinate, burning or pain with urination, blood in the urine, or fever, notify your healthcare team right away.
Intravesicular chemotherapy may cause burning with urination, cramps, and diarrhea.
In some rare cases, mitomycin can cause a decrease in your blood counts. This can include white blood cells (important for fighting infection), platelets (for blood clotting), and red blood cells. This is more likely to occur when the medication is given after surgery, and there is an injury in the bladder (cuts, tears), allowing the drug to be absorbed into the bloodstream. However, it can occur with any instillation.
References
[1] Ashish Ranjan. “Clinical Efficacy of Neoadjuvant Intravesical Mitomycin-C Therapy Immediately Before Transurethral Resection of Bladder Tumor in Patients With Nonmuscle-invasive Bladder Cancer: Preliminary Results of a Prospective, Randomized Phase II Study. Letter.” Journal of Urology (2023).
[2] R Hurle. “Intravesical instillation of mitomycin-C in 242 patients with superficial bladder cancer at high risk of recurrence: long-term results.” Urologia Internationalis 61 4 (1998): 220–6.
- Related articles
- Related Qustion
Denosumab is a type of targeted drug called a monoclonal antibody. It is also known as Prolia and Xgeva.....
Apr 12,2024APIUranium nitrides can be used as nuclear fuel and anti-corrosion coating for uranium metal. This article will show the crystal structure of some different type of uranium nitrides.....
Apr 12,2024Inorganic chemistryMitomycin C
50-07-7You may like
- Crystal Structure of Manganese selenide
May 23, 2024
- What is the Crystal Structure of Titanium boride?
May 23, 2024
- What is the crystal structure of beryllium hexaboride?
May 23, 2024
- Mitomicin C
-

- $0.00 / 25Kg/Bag
- 2024-05-23
- CAS:50-07-7
- Min. Order: 2Kg/Bag
- Purity: 99% up, High Density
- Supply Ability: 20 tons
- Mitomycin C
-

- $0.00 / 25kg
- 2024-04-12
- CAS:50-07-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000ton
- Mitomycin C
-

- $0.00 / 1KG
- 2024-03-16
- CAS:50-07-7
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg