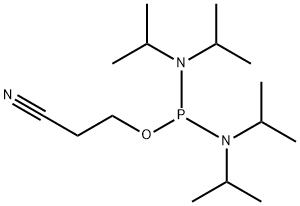
2-Cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite synthesis
- Product Name:2-Cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite
- CAS Number:102691-36-1
- Molecular formula:C15H32N3OP
- Molecular Weight:301.41

3-((dichlorophosphaneyl)oxy)propanenitrile could synthesize 2-Cyanoethyl N, N, N', N'-tetraisopropylphosphorodiamidite with diisopropylamine. This method is inexpensive manner using a two-step, one-pot procedure and purified by vacuum distillation[2].
Yield:102691-36-1 88.5%
Reaction Conditions:
in tetrahydrofuran at -10 - 20; for 73.5 h;
Steps:
1 Example 1; Preparation and Stabilization of 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphoro-diamidite (PDA)
Example 1; Preparation and Stabilization of 2-Cyanoethyl- N, N, N', N'-tetraisopropylphosphoro-diamidite (PDA) 2-(cyanoethoxy) dichlororphosphine (CDP) can be easily prepared by various processes described in the prior art. For example, the reaction of phosphorous trichloride (PCl3) with either 2-cyanoethanol or trimethylsilyloxypropionitrile (TMSOP) gives CDP in good yield. If desired, the product can be purified with careful vacuum distillation. The CDP used in this example, and Example 2 was obtained by reacting PCl3 with TMSOP in a molar ratio of 2: 1 in acetonitrile at 5°C. Upon completion of the reaction, the solvent and the excess POS were removed by distillation. The crude product was further purified by vacuum distillation, and gave CDP with greater than 98% purity. A solution comprising 1.80 moles of freshly distilled 2-(cyanoethoxy) dichlorophosphine (CDP) (310 grams) in 2.9 Kg of tetrahydrofuran (THF) as solvent was stirred and cooled to -12 °C under a nitrogen blanket and then treated with 8.12 moles (820 grams) of diisopropylamine over a period of ninety minutes, maintaining a temperature of-10 °C. Stirring of the slurry was continued at ambient temperature for a period of 72 hours, at which time examination by 31P NMR showed no reaction intermediates remaining. The slurry was filtered through a sintered glass filter to remove diisopropylamine hydrochloride (DIPA. HCl) solids. The clear filtrate was passed through a column of 350 grams of Brockmann activated neutral alumina that had been dried at 165°C at less than 1 Torr for 16 hours. The filtrate was concentrated on a rotary evaporator at a maximum bath temperature of 45°C. The distilled THF was used to rinse the DIPA. HCI and the alumina to collect additional product, which was combined with the first pass product and then concentrated until the vacuum reached 3 Torr. The yield of the pale yellow syrup was 485 grams (88.5% of theoretical) and the initial assay by 31P NMR was 99.7%. Table 1 shows a comparison of the stability of this material (A) vs. a sample prepared in the same manner, but without the alumina treatment (B).
References:
RHODIA, INC. WO2003/106468, 2003, A1 Location in patent:Page 6-7
108-18-9
353 suppliers
$10.00/1g

109-78-4
349 suppliers
$10.00/1g

102691-36-1
363 suppliers
$6.00/1g
56183-63-2
120 suppliers
$16.80/250MG

109-78-4
349 suppliers
$10.00/1g

102691-36-1
363 suppliers
$6.00/1g

108-18-9
353 suppliers
$10.00/1g

102691-36-1
363 suppliers
$6.00/1g
