
Ethyl 4-(1-hydroxy-1-methylethyl)-2-propyl-imidazole-5-carboxylate synthesis
- Product Name:Ethyl 4-(1-hydroxy-1-methylethyl)-2-propyl-imidazole-5-carboxylate
- CAS Number:144689-93-0
- Molecular formula:C12H20N2O3
- Molecular Weight:240.3


74-83-9
2 suppliers
$28.30/1ML

144689-94-1
271 suppliers
$8.00/5g

144689-93-0
474 suppliers
$16.00/5g
Yield:144689-93-0 97.5%
Reaction Conditions:
with iodine;magnesium in dichloromethane at 15 - 25; for 2 h;
Steps:
4
Methyl bromide gas (35 g, 0.3686 mol) was passed through a dry solution of tetrahydrofuran (100 mL) and a small portion (5 to 10 mL) of this solution was added dropwise to a solution of magnesium strip (8 g, 0.33 mol) The solution of iodine (0.02g) and dry tetrahydrofuran (50mL) was stirred at room temperature. After the reaction was initiated, the solution of the remaining methyl bromide in THF was slowly added dropwise to keep the reaction slightly boiling. After the addition was complete, the reaction was continued for 3h until the magnesium strip disappeared. Get the corresponding Grignard reagent;The imidazole diesters of the compounds of the formula II 15g (0.059mol) was dissolved in dry dichloromethane (50mL), slowly added dropwise to the homemade format reagents, the dropping period, maintaining the temperature of the reaction system below 15 °C, after the addition was complete, the control The reaction was continued stirring at a temperature of 15 ~ 25 °C for 2h, after the reaction was cooled to 0 °C, further diluted with ethyl acetate (150mL), and then saturated amine chloride solution (100mL) was slowly added dropwise The process of maintaining the system temperature below 10 °C, dropping completed, the organic layer was collected by stratification, washed with saturated sodium chloride solution (30mLX3), and then dried over anhydrous sodium sulfate, and then concentrated under reduced pressure to remove the solvent To give a residue. The resulting residue was recrystallized from isopropyl ether-n-hexane solvent, filtered and dried to give a white powdery solid 4- (1-hydroxy-1-methylethyl) -2-propyl- 1H-imidazole-5-carboxylate (13.8 g, yield: 97.5% , HPLC: 99.7%)
References:
CN104177296,2017,B Location in patent:Paragraph 0048; 0049; 0052; 0053
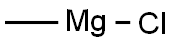
676-58-4
277 suppliers
$15.00/25ml

144689-94-1
271 suppliers
$8.00/5g

144689-93-0
474 suppliers
$16.00/5g
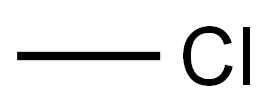
74-87-3
195 suppliers
$18.50/48622

144689-94-1
271 suppliers
$8.00/5g

144689-93-0
474 suppliers
$16.00/5g

64-17-5
699 suppliers
$10.00/50g

849206-42-4
21 suppliers
inquiry

144689-93-0
474 suppliers
$16.00/5g

75-16-1
266 suppliers
$12.00/10ml

144689-94-1
271 suppliers
$8.00/5g

144689-93-0
474 suppliers
$16.00/5g