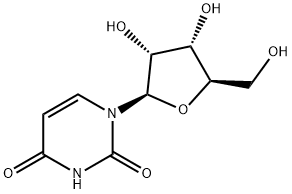
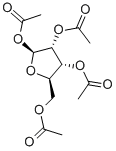
2',3',5'-Tri-O-acetyluridine synthesis
- Product Name:2',3',5'-Tri-O-acetyluridine
- CAS Number:4105-38-8
- Molecular formula:C15H18N2O9
- Molecular Weight:370.31

Reference: Zhou, X. X.; Welch, C. J.; Chattopadhyaya, J. Pyridyl groups for protection of the imide functions of uridine and guanosine. Exploration of their displacement reactions for site-specific modifications of uracil and guanine bases. Acta Chemica Scandinavica, Series B: Organic Chemistry and Biochemistry. Volume B40. Issue 10. Pages 806-16. Journal. (1986).
Yield:4105-38-8 74%
Reaction Conditions:
with sodium hydroxide in water at 20; pH=8; for 4 h;
Steps:
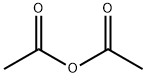
General Nucleoside Acetylation Protocol
General procedure: Nucleoside/nucleotide (2; 100 mM) and N-acetyl imidazole (1a;10 equiv) were dissolved in water (pH 8; adjusted with 4 MNaOH). The solution was incubated at r.t. for 4 h, and NMR spectra were periodically acquired. The product was purified byreverse-phase (C18) flash coumn chromatography (eluted at pH4 with 100 mM NH4HCO2/MeCN = 98:2 to 80:20). The fractions containing 5 were lyophilised to yield a white powder.
References:
Fernández-García, Christian;Powner, Matthew W. [Synlett,2017,vol. 28,# 1,art. no. ST-2016-S0507-C,p. 78 - 83] Location in patent:supporting information

55003-25-3
15 suppliers
inquiry

4105-38-8
255 suppliers
$7.00/1g