
2,6-Difluoropyridine-3-boronic acid synthesis
- Product Name:2,6-Difluoropyridine-3-boronic acid
- CAS Number:136466-94-9
- Molecular formula:C5H4BF2NO2
- Molecular Weight:158.9

1513-65-1
339 suppliers
$6.00/5g
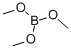
121-43-7
344 suppliers
$13.00/25mL

136466-94-9
213 suppliers
$8.00/250mg
Yield:136466-94-9 94%
Reaction Conditions:
Stage #1:2,6-difluoro pyridine with n-butyllithium;lithium diisopropyl amide in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2:Trimethyl borate in tetrahydrofuran at 20; for 1 h;
Steps:
2’,6’-Difluoro-2,3’-bipyridine, L2
L2 was synthesized according to literature procedures [S1,S2] with some modifications. In around-bottomed flask, lithium diisopropylamide (LDA) solution was prepared as follows: THF (20mL) solution of diisopropylamine (3.4 mL) was slowly added n-butyl lithium (1.67 M in n-hexane,24 mmol, 14 mL) at 0 C, then was stirred at the same temperature for 20 min. In anotherround-bottomed flask, THF (20 mL) solution of 2,6-difluoropyridine (20 mmol, 1.8 mL) was stirredat -78 C and was added LDA solution prepared above via a cannula. After stirring at -78 C for 30min, the reaction mixture was added THF (10 mL) solution of trimethyl borate (24 mmol, 2.5 g, 2.7mL), then warmed to ambient temperature over 1 h. The reaction was quenched by adding 2Maqueous sodium hydroxide (40 mL). Separated aqueous layer was neutralized (pH 8) by adding 4Mhydrochloric acid and extracted with ethyl acetate (organic layer A). Re-adding 4M hydrochloricacid to the aqueous layer to pH 6.5 afforded insoluble organic materials. Repeated extraction withethyl acetate for three times gave organic layer B. Re-adding 4M hydrochloric acid to the aqueouslayer to pH 4 followed by repeated extraction with ethyl acetate for three times gave organic layer C.The combined organic layer (B and C) was dried over anhydrous sodium sulfate, and concentrated invacuo. 2,6-difluoropyridine-3-boronic acid was obtained in 94% yield (3.0 g, 19 mmol) as theresidue. This compound was used for the next step without further purification.
References:
Takahira, Yusuke;Murotani, Eisuke;Fukuda, Keiko;Vohra, Varun;Murata, Hideyuki [Journal of Fluorine Chemistry,2016,vol. 181,p. 56 - 60] Location in patent:supporting information

1513-65-1
339 suppliers
$6.00/5g
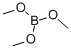
121-43-7
344 suppliers
$13.00/25mL
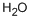
7732-18-5
458 suppliers
$12.69/100ml

136466-94-9
213 suppliers
$8.00/250mg

1513-65-1
339 suppliers
$6.00/5g

136466-94-9
213 suppliers
$8.00/250mg

1513-65-1
339 suppliers
$6.00/5g
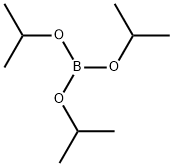
5419-55-6
369 suppliers
$12.00/5g
7732-18-5
458 suppliers
$12.69/100ml

136466-94-9
213 suppliers
$8.00/250mg

1513-65-1
339 suppliers
$6.00/5g
121-43-7
344 suppliers
$13.00/25mL
108-18-9
352 suppliers
$10.00/1g

136466-94-9
213 suppliers
$8.00/250mg