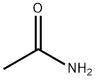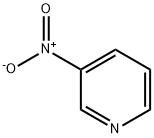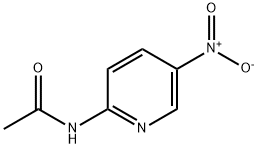
2-Acetamido-5-nitropyridine synthesis
- Product Name:2-Acetamido-5-nitropyridine
- CAS Number:5093-64-1
- Molecular formula:C7H7N3O3
- Molecular Weight:181.15

4214-76-0
517 suppliers
$6.00/10g
75-36-5
559 suppliers
$17.92/100G

5093-64-1
164 suppliers
$11.00/1g
Yield: 80.1%
Reaction Conditions:
with pyridine in tetrahydrofuran at 0 - 20; for 2 h;
Steps:
155.1
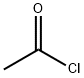
Example 155; N-{6-[Acetyl(methyl)amino]pyridin-3-yl]-4-(3-phenyl-1,2,4-thiadiazol-5-yl)piperazine-1-carboxamide; (1) N-(5-Nitropyridin-2-yl)acetamide; To a solution of 2-amino-5-nitropyridine (13.9 g, 100 mmol) and pyridine (24 ml, 300 mmol) in tetrahydrofuran (300 ml) was added dropwise acetyl chloride (10. 7 ml, 150 mmol) with ice-cooling, the mixture was stirred at room temperature for 1 hour. To the mixture was added dropwise acetyl chloride (10.7 ml, 150 mmol) and the mixture was stirred at room temperature for 1 hour. The reaction mixture was poured into ice-water and the desired product (14.5 g, 80.1%) as a solid was collected by filtration. 1H-NMR (CDCl3) δ; 2.40 (3H, s), 3.57 (3H, s), 7.99 (1H, d, J = 9.0 Hz), 8.44 (1H, dd, J = 3.0, 9.0 Hz), 9.25 (1H, d, J = 3.0 Hz).
References:
Takeda Pharmaceutical Company Limited EP1813606, 2007, A1 Location in patent:Page/Page column 85

4214-76-0
517 suppliers
$6.00/10g
108-24-7
5 suppliers
$14.00/250ML

5093-64-1
164 suppliers
$11.00/1g

4214-76-0
517 suppliers
$6.00/10g

29958-14-3
140 suppliers
$17.00/1g

5093-64-1
164 suppliers
$11.00/1g

4214-76-0
517 suppliers
$6.00/10g
127-19-5
802 suppliers
$5.00/25g

5093-64-1
164 suppliers
$11.00/1g